Prostate cancer represents the third leading cancer for men with >80% expected to be diagnosed with prostate cancer by the age 80. Worldwide, it accounts for ~350,000 deaths yearly with nearly 30,000 in the United States (~170,000 diagnosed/year). The 5-year survival rate varies considerably ranging from close to ~100% for loco-regional cases to mere ~29% for distant metastatic cases.
Paralleling the situation with breast cancer, prostate cancer may occur in secretory glands and is regulated by hormones--i.e. the male hormone testosterone. Most of adenocarcinoma (accounts for 95-99% of prostate cancers) occur in acinar (secreting cells) than ductal (tubular cells) part. Recent researches suggest that the conversion of prostate gland cells into a cancerous state may entail mutation in genes such as ETS (transcription factor) in early stage, and in PTEN (occur in ~70% of patients), RB (decreases survivability 3-fold), p53 (tumor suppressor), ATM, CHK2 (DNA damage checkpoint), MMR, BRCA1/2 (DNA repair), etc. in later stages involving metastasis (Arora et al., 2018).
A commonly used biomarker in the initial screening of prostate cancer is PSA (prostate specific antigen). It represents a serine protease secreted by the prostate gland, which is normally present at a low level in the blood. PSA may exist in either a 'free' state or in complex with serum proteins (ex. alpha 1-antichymotrypsin). The elevation in the PSA level is a risk factor for prostate cancer though certain subtypes (ductal prostate cancer, neuroendocrine tumor, small cell carcinoma, etc.) may occur in the absence of such increase. Other conditions like prostatitis (inflammation) or benign hyperplasia may increase the PSA level, resulting in false positives. Thus, further diagnosis via the histological analysis of biopsied specimens is necessary to confirm positivity.
The staging of prostate cancer (from T1 to T4) incorporates Gleason scoring system (2 to 10 with higher score representing aggressive cancer with poor outcome based on histopathological examination of the tumor). At T2 stage, the tumor volume is larger than at T1; however, tumors are confined to within the prostate in both stages. In T3 stage, tumors have invaded to nearby organs such as bladder; in T4, it has metastasized to distant organs such as the bone or nearby lymph nodes, which may be incurable despite the treatment. The onset of 'biochemically recurrent' prostate cancer is indicated by the rise in PSA level despite the inability to monitor them through imaging--ex. metastatic prostate cancer. T1 stage prostate cancer may remain stable for a considerable period but treatment options include prostectomy (surgically removing prostate), radiotherapy, etc.
As with breast cancer, anti-hormone therapy represents a significant part of the current treatment strategies for prostate cancer. The testosterone level could be lowered (~95%) through further surgery or via treatment with drugs that block upstream signaling events occurring at the level of the brain (ex. lutenizing hormone releasing hormone agonist, or its receptor antagonist). Despite the lowering of testosterone level, cancer may still grow (He et al., 2020).
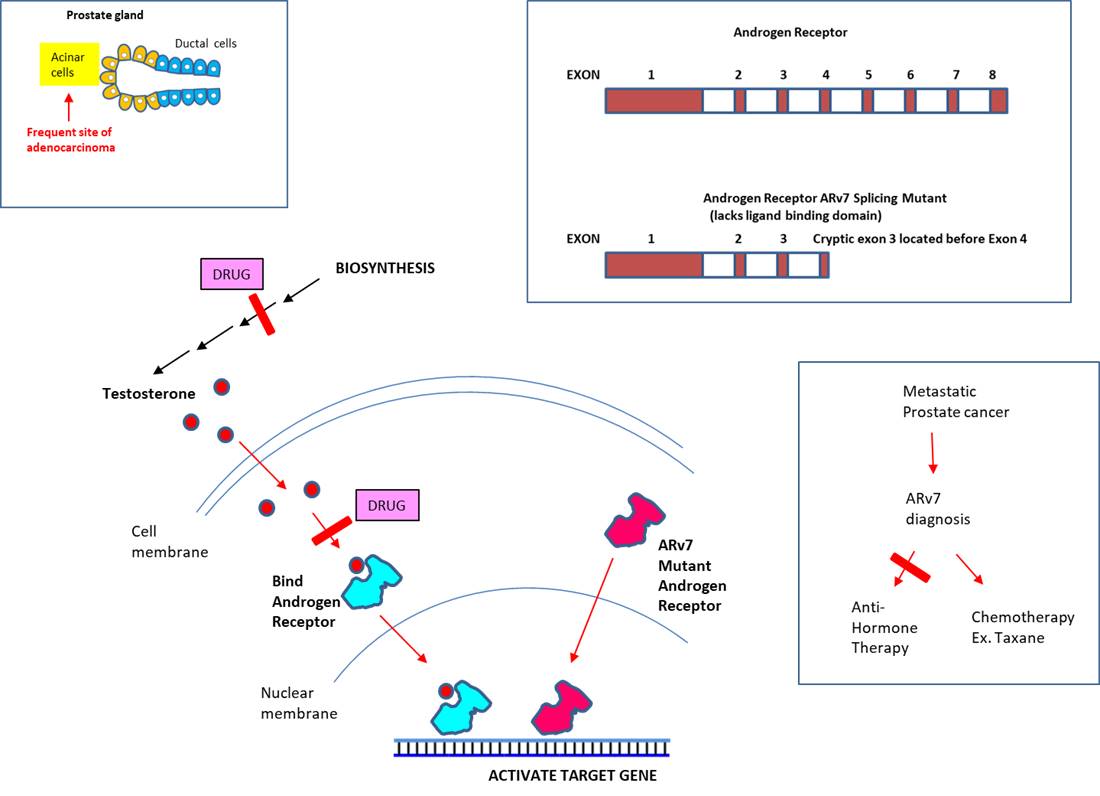
Mechanistically, testosterone freely crosses the cell membrane to interact with its receptor (a nuclear receptor called 'androgen receptor') in the cytoplasm. Binding of the ligand to the receptor activates (alters conformation) and dissociates from Hsp protein to enter the nucleus, where it transcriptionally activates >200 genes (including the PSA gene) by binding to the enhancer of the target gene's promoter (Jin et al., 2013).
Testosterone could also be produced by adrenal gland (situated above kidney) or prostate tumor tissue. Other anti-hormone therapeutics (androgen-deprivation therapy: ADT) target events concerning testosterone directly. Analogous to breast cancer chemopreventive therapeutics targeting estrogen biosynthesis pathway, abiraterone acetate is converted to its metabolite abiraterone, which inhibits the biosynthesis of testosterone. Another strategy is to counter testosterone's ability to activate its cognate receptor, i. e. 'androgen receptor'. Enzaluatemide is a novel anti-androgen drug, which is used to treat 'metastatic CRPC' (median survival of 9-13 months). In addition to its antagonizing activity, the latter may prevent binding of the receptor to DNA or co-activators.
Eventually, most prostate cancers become insensitive to anti-hormone therapy. Diverse types of mutations have been reported: for instance, switching the antagonist drug to agonist, or rendering the androgen receptor responsive to irrelevant hormones, etc. It may also occur through mutations that allow the receptor to be constitutively active without binding to testosterone. Among the splicing mutants, ARv7, which lacks the ligand-binding domain, was the only variant detected at the protein level (Wadosky et al., 2017). Though the ARv7 mutation is present in ~1% of early stage cancer, nearly 75% of metastatic prostate cancers harbor the splicing mutation (Zhang et al., 2020). The landmark study by investigators at the Johns Hopkins School of Medicine (USA) demonstrated the association between ARv7 and the resistance to anti-hormone therapy (Antonarakis et al., 2014). Further clinical study showed that ARv7-positive patients who received Taxane therapy (ex. Taxol derivative) had better outcome than those who received anti-hormone therapy (abiraterone or enzalutamide) (Antonarakis, 2015). Finally, another mechanism through which the insensitivity to anti-hormone therapy could occur is through its ability to grow independent of testosterone.
As a result, ARv7 has emerged as an important therapy biomarker for advanced stage prostate cancer. To detect ARv7 mRNA, RT-PCR could be performed on circulating tumor cells (CTCs) isolated from blood, which is more accessible than tumor biopsies--ex. Qiagen Adna Test ProstateCancerPanel AR-V7 Test. Alternatively, CTCs could be assayed for the presence of ARv7 protein in the cell nucleus--ex. Oncotype DX AR-V7 Nucleus Detect Test. As CTCs are rare, detecting the ARv7 mRNA in blood, urine, saliva or other liquid biopsies as circulating cell-free nucleic is being investigated extensively (Boerrigter et al., 2019). One disadvantage of using tumor biopsies is that it may yield heterogeneous results depending on the specific loci being assayed. In light of persisting Covid-19 pandemics, a point-of-care (POC) diagnostic method capable of detecting ARv7 would be desirable.
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. For medicinal chemistry, it specializes in peptide synthesis, characterization, modification, purification to generate various peptide-based building blocks as well as pharmaceutical intermediates—in addition to peptide libraries, peptide arrays, peptidomimetics. Antibody purification, characterization/quantification, modification and labeling are also offered. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
References
Antonarakis ES, Lu C, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371:1028-38 (2014). PMID: 25184630
Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol 1:582-91 (2015). PMID: 26181238
Arora K, Barbieri CE. Molecular Subtypes of Prostate Cancer. Curr Oncol Rep. 1;20:58 (2018). PMID: 29858674
Boerrigter E, Groen LN, et al. Clinical utility of emerging biomarkers in prostate cancer liquid biopsies. Expert Rev Mol Diagn. 20:219-230 (2020). PMID: 31577907
He L, Fang H, et al. Metastatic castration-resistant prostate cancer: Academic insights and perspectives through bibliometric analysis. Medicine 99:e19760 (2020). PMID: 32282738
Jin HJ, Kim J, et al. Androgen receptor genomic regulation. Transl Androl Urol. 2:157-177 (2013). PMID: 25237629
Wadosky KM, Koochekpour S. Androgen receptor splice variants and prostate cancer: From bench to bedside. Oncotarget 8:18550-18576 (2017). PMID: 28077788
Zhang T, Karsh LI, et al. Androgen Receptor Splice Variant, AR-V7, as a Biomarker of Resistance to Androgen Axis-Targeted Therapies in Advanced Prostate Cancer. Clin Genitourin Cancer 18:1-10 (2020). PMID: 31653572