To date, hundreds of different species of cornaviruses have been identified, which infect a variety of organisms. For the poultry industry, a significant economic loss has been associated with avian coronavirus IBV, which causes avian infectious bronchitis. IBV infects a variety of tissues including respiratory tract, digestive system, reproductive system, kidney, etc (Niesters et al., 1986). Despite the availability of vaccines, its containment is beset by the occurrence of random mutants, which give rise to new variants that escape ‘herd immunity’.
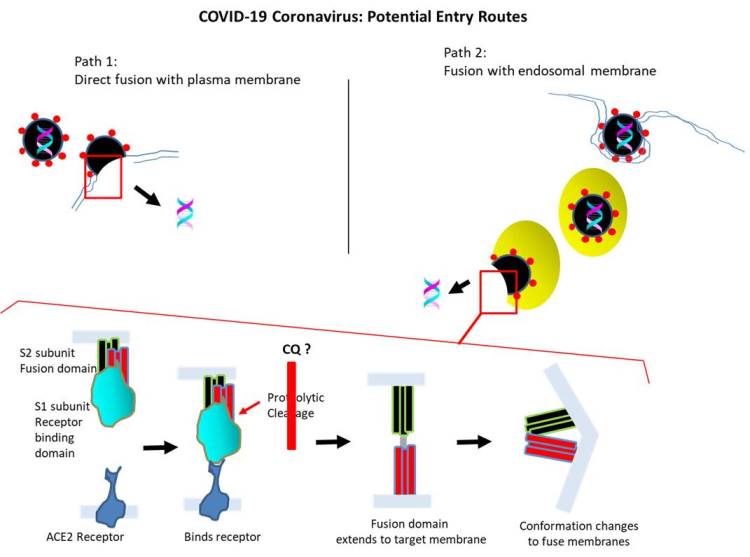
The current pandemic causing severe respiratory dysfunction is caused by COVID-19 coronavirus. During infection, spike protein (S) plays a critical role as its interaction with a cellular receptor initiates a series of events leading to its internalization. For COVID-19, the receptor binding domain located in S1 subunit of S protein may bind to angiotensin-converting enzyme 2 (ACE2) expressed by specific cells in bronchi, lung, tongue, etc. (which is also utilized by SARS and HCoV-NL63 human coronaviruses for cell entry) (Walls et al., 2020).
As a component of the renin-angiotensin system that regulates blood pressure, angiotensin I is converted to angiotensin II by ACE (angiotensin converting enzyme), which constricts blood vessels to reduce flow. This is countered by ACE2 that hydrolyzes angiotensin II. The binding of ACE2 by COVID-19 spike protein, was suggested to downregulate ACE2 (Bombardini et al., 2020), which may decrease the flow of pulmonary artery into the lung, hence affecting the level of oxygenated blood. This may exacerbate the negative effects brought on by cytokine release syndrome (also known as ‘cytokine storm’) due to hyperinflammation, which causes ARDS (acute respiratory distress syndrome), that a minor subset of COVID-19 infected people experience.
The cell entry of COVID-19 requires the fusion of vial envelope with the cell membrane. Whether the fusion occurs at the plasma membrane or endosomal membrane following endocytic uptake (or both) has not been clearly delineated. Following the binding of S protein to ACE2 receptor, it undergoes a proteolytic cleavage to separate the receptor binding domain from the fusion domain located in S2 subunit. This is thought to be mediated by TMPRRS2 protease (or possibly others, ex. endosomal proteases that are active at lower pH).
Further, an additional cleavage occurs at a distinct site to expose the ‘fusion peptide’ comprised of hydrophobic residues. The liberated fusion domain inserts into the targeted cellular membrane to form a ‘pre-hairpin’ structure using the fusion peptide. Then, two heptad repeats (HR1 and HR2) residing in S2 subunit join to form an antiparallel 6-helix bundle, which serves to pull back on the viral and cellular membranes, allowing tem to fuse. The fusion creates a channel through which the virus capsid (protein sheath containing its RNA genome) can enter the cell interior.
Chloroquine (CQ) has been used extensively to manage malaria though its efficacy is limited by the emergence of resistant strains. The plasmodium malaria invades red blood cells and catabolizes hemoglobin in its food vacuoles (acidic), with the resultant byproduct (heme) being detoxified. Chloroquine, which permeates through diffusion, becomes trapped inside the vacuole upon protonation, and disrupts the detoxification of heme, leading to cell lysis (Lin et al., 2015).
Likewise, chloroquine accumulates in late endosomes (or lysosomes) and increases their pH. This may potentially interfere with the function of endosomal proteases that activate spike proteins for fusion with endosomal membrane for COVID-19 entry. This view (i.e interference with endocytic uptake) is indirectly supported by the finding that chloroquine prevents the receptor mediated endocytosis of nanoparticles (of similar size as COVID-19 virus) by inhibiting PICALM (phosphatidyl inositol binding clathrin assembly protein) that regulates endocytosis (Hu et al., 2020). To explore, the potential efficacy of chloroquine in preventing COVID-19 pathogenesis is being assessed through numerous (as many as ~50) clinical trials that are currently underway. Nevertheless, chloroquine (or its derivative hydroxychloroquine) may cause side effects (ex. may affect vision, cardiac problems).
Chloroquine may interfere with additional steps in endocytic trafficking. Of interest is the finding that chloroquine may block the glycosylation of ACE2 receptor to interfere with SARS infection (Vincent et al., 2005). Endocytic trafficking is also exploited for cell entry by oncogenic viruses such as human papilloma virus (HPV), which causes head and neck cancer, cervical cancer, and other malignancies (~5% of all cancers globally).
Bio-Synthesis, Inc.—with its capacity to provide siRNA oligonucleotides with unsurpassed specificity and stability--is committed to supporting COVID-19 therapy initiatives. Further, the key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, it provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. Bio-Synthesis, Inc. also specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology that we offer provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power was especially useful for diagnosis (ex. FISH using DNA probe). More importantly, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides for clinical application.
https://www.biosyn.com/oligonucleotide-modification-services.aspx
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
References
Bombardini T, Picano E. Angiotensin-Converting Enzyme 2 as the Molecular Bridge Between Epidemiologic and Clinical Features of COVID-19. Can J Cardiol. Mar 29. pii: S0828-282X(20)30299-3. (2020) PMID: 32299780 doi: 10.1016/j.cjca.2020.03.026.
Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 15:247-249 (2020). PMID: 32203437 doi: 10.1038/s41565-020-0674-9.
Lin JW, Spaccapelo R, Schwarzer E, Sajid M, Annoura T, Deroost K, et al. Replication of Plasmodium in reticulocytes can occur without hemozoin formation, resulting in chloroquine resistance. J Exp Med.212:893-903 (2015). PMID: 25941254 doi: 10.1084/jem.20141731.
Niesters HG, Lenstra JA, Spaan WJ, Zijderveld AJ, Bleumink-Pluym NM, Hong F, et al. The peplomer protein sequence of the M41 strain of coronavirus IBV and its comparison with Beaudette strains. Virus Res. 5:253-63 (1986). PMID: 2429473 PMCID: PMC7134181 DOI: 10.1016/0168-1702(86)90022-5
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2:69 (2005). PMID: 16115318 doi: 10.1186/1743-422X-2-69.
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181:281-292.e6. (2020) PMID: 32155444 doi: 10.1016/j.cell.2020.02.058. Epub 2020 Mar 9.