The current strategy to manage the COVID-19 pandemic relies heavily on the accuracy of the existing diagnostic methods to detect the presence of its genomic RNA. However, even with RT-qPCR, it yields little information regarding the functionality, i.e. the capacity to undergo replication. Nor does it provide insight regarding the infectivity of the virus. (Further, the sequence of the amplified product is seldom analyzed to confirm it's derived from COVID-19--as qPCR merely monitors the increase in fluorescence emitted by the probe). To address the above, the investigators at the University of Manitoba (Canada) examined the relationship between the RT-PCR outcome versus infectivity (monitored the ability to grow COVID-19 in cell culture in lab, i.e. the "true" gold-standard). For this, patient samples obtained at various time points since the onset of symptoms were examined. Intriguingly, the ability to infect was highest on day 3 (since the onset of symptom) and declined 32% for every 1-unit increase in Ct thereafter. Though infectivity steadily declined and was no longer observed after 7th day, the genomic RNA was detected by RT-PCR well beyond 12th day (Jefferson et al, 2020'; Bullard et al., 2020) (Fig 1A). The results corroborate that RT-PCR may not be a reliable indicator of COVID-19 infectivity.
Nevertheless, the diagnostic tests were carried out on a massive scale in recent months, which resulted in a significant rise in the count of the COVID-19 infected individuals. The daily reports concerning the elevating count globally have prompted some to ask what its real impact was. Yet, the impact has been difficult to assess as the count of COVID-19 cases may have included those who are asymptomatic, those who are at low-risk, ones who have recovered, those who have died, those who have died due to other causes, and potentially the results obtained using methods that cross-react with other coronaviruses, diagnostic kits revoked by FDA, diagnostic assays with high rate of false negativity/positivity, etc.
As a result, to assess the impact directly, the focus has shifted towards the number of deaths caused by COVID-19. There are two different ways to determine mortality rate: CRF (case fatality rate) refers to the proportion of deaths when compared to the total number of cases diagnosed with COVID-19 disease) whereas IFR (infection fatality rate) refers to the number of deaths per total number of infected cases (which may include healthy asymptomatic individuals). According to the Johns Hopkins coronavirus resource center, the current CRF for United States is ~3.0%. Though this statistic is used commonly amongst physicians, CRF figures do not take into account of those clinically undetected (i.e. those who display atypical or asymptomatic to very mild symptoms).
Meta-study refers to combining the results from the previously conducted individual studies (ex. clinical trials) to arrive at a point with greater statistical power. Meta-analysis conducted on published reports, government documents, etc. from various global regions yielded IFR of ~0.68% (with 0.53-0.82% range) for COVID-19 (Meyerowitz-Katz et al., 2020). This is comparable to IFR of 0.63% reported for the state of Arizona (USA) (August 2000; https://www.statnews.com/2020/08/24/infection-fatality-rate-shows-covid-19-isnt-getting-less-deadly/ ). A report from Stanford University (USA) showed a median IFR value of 0.27% IFR for 32 global locations (though higher for 'extreme hotbed locations') (Ioannidis, 2020). The latest figure from CDC says: 0.0002 (or 0. 02% for 20-49 y), 0.005 (or 0.5% for 50-69 y), 0.054 (or 5.4% for >70y; https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html#table-1 ). in contrast, IFR for seasonal influenza virus is estimated ~0.1%.
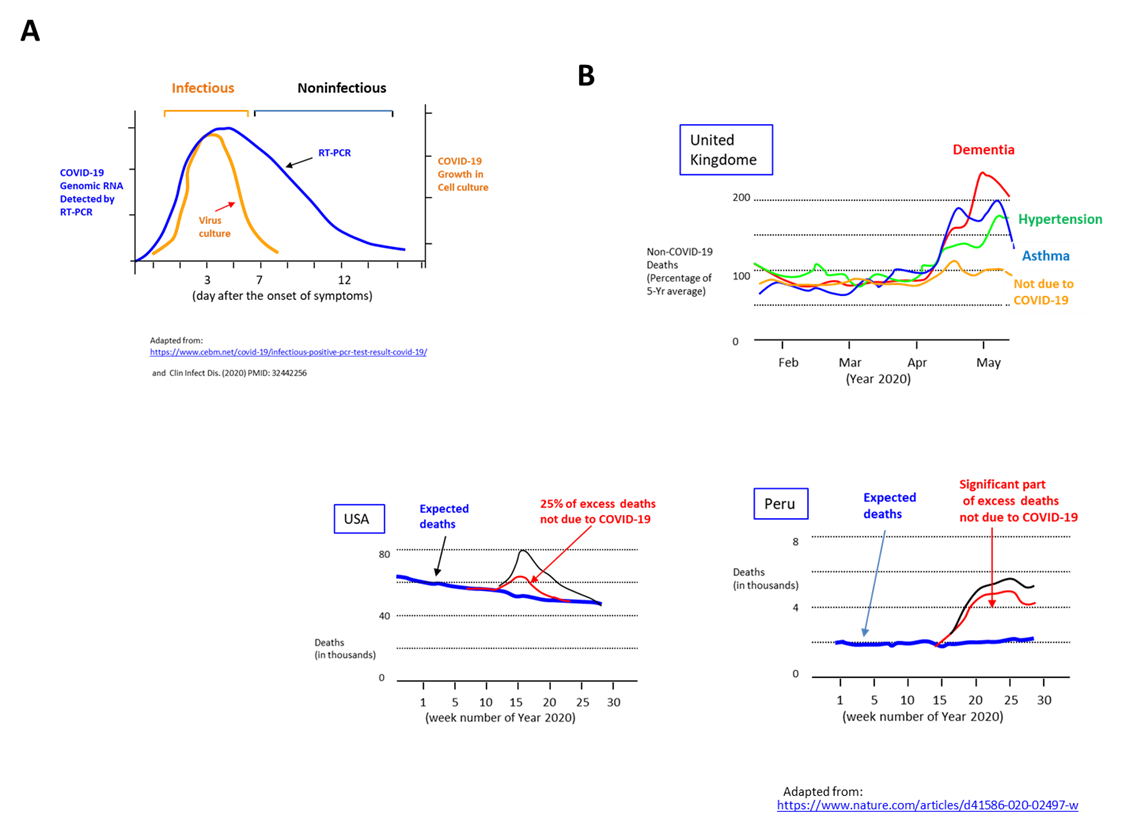
To gauge the true burden of COVID-19, the extent of 'excess mortality' was examined. It is obtained by comparing all deaths in a given period with the number of predicted deaths based on average of preceding years (ex. 5 years). But it turns out a significant fraction of excess deaths was not attributed to COVID-19, i.e. 25% in the case of United States and 74% in Peru (from March through June 2020) (Fig. 1B). Further, the remaining fraction may need to be divided into those who died due to COVID-19 versus those who died due to unrelated causes (despite being infected). The excess deaths may also include deaths caused by lockdown indirectly (ex. patients skipping hospital visits, domestic violence, exacerbated mental conditions), as documented by a sharp increase in deaths due to asthma, diabetes, hypertension, dementia, Alzheimer's disease in United Kingdom (Viglione, 2020).
As those with underlying conditions are more susceptible to COVID-19 associated death, investigators at Sloan Kettering Memorial Cancer Center (USA) investigated the impact of various treatment modalities on >400 cancer patients exhibiting COVID-19 symptoms (Robilatti et al., 2020). In addition to conventional treatments, immunotherapy is increasingly used to manage melanoma, lung cancer and several other cancers. The recently introduced therapeutic agent called 'immune checkpoint inhibitor' (ex. PD-1 blockade) is designed to increase autoimmunity against tumor. The study showed that lung cancer and other solid tumor patients receiving immune checkpoint inhibitors had higher frequency of hospitalization and severe respiratory illness from COVID-19. In contrast, chemotherapy and surgery did not pose a greater risk.
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. For medicinal chemistry, it specializes in peptide synthesis, characterization, modification, purification to generate various peptide-based building blocks as well as pharmaceutical intermediates—in addition to peptide libraries, peptide arrays, peptidomimetics. Antibody purification, characterization/quantification, modification and labeling are also offered. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/peptide-synthesis.aspx
https://www.biosyn.com/tew/Potential-Peptide-Targets-for-a-COVID-19-Vaccine.aspx
References
Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. (2020) PMID: 32442256
Ioannidis J. The infection fatality rate of COVID-19 inferred from seroprevalence data. medRxiv (2020) doi: https://doi.org/10.1101/2020.05.13.20101253
Jefferson T, Spencer E, Brassey J, Heneghan C. Viral cultures for COVID-19 infectivity assessment. medRxiv (2020) doi: https://doi.org/10.1101/2020.08.04.20167932
Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection-fatality rates. medRxiv (2020) doi: https://doi.org/10.1101/2020.05.03.20089854
Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 26:1218-1223 (2020). PMID: 32581323
Viglione G. How many people has the coronavirus killed? Nature. 585:22-24 (2020) PMID: 32873974