Most naturally occurring constrained peptides maintain the rigidity of the peptide framework by disulfide bond or N- to C-termini backbone cyclization. Commonly known antimicrobial peptides like Gramidicin S, Bacitracin and Polymyxin B are an example of backbone cyclic peptides that are used clinically (Table 1).
Table 1: Naturally occurring backbone cyclized peptides
|
Peptide
|
Structure
|
|
Gramicidin S
Gramicidin S is a cyclodecapeptide, constructed as two identical pentapeptides joined head to tail [cyclo(-Val-Orn-Leu-D-Phe-Pro-)2] forming a ring structure composed of five different amino acids. Gramicidin S is synthesized by gramicidin S synthetase.
PBD ID , 1TK2, Gramicidin.
|
Cyclic (L-dF-P-V-Orn-L-dF-P-V-Orn)
|
|
Bacitracin
The mature antibiotic is formed by the cyclodehydration of Cys-2 with the backbone to form a thiazoline ring and forming a lariat structure via an isopeptide bond between the ε-amino group of Lys-6 and the peptide’s C terminus.The structure of the ternary complex of bacitracin, zinc, and geranyl-pyro-phosphate is shown here.
PBD ID 4K7T , Bacitracin.
|
I-C-L-dE-L-cyclic (K-dOrn-I-dF-H-dD-N)
|
|
Polymyxin E
Polymyxin disrupts the outer membrane of Gram negative bacteria. The α- and γ-diaminobutyric acid of a positively charged polymyxin B forms an electrostatic interaction with the phosphate groups of a negatively charged lipid A on the outer membrane of a Gram negative bacterium. Calcium and Magnesium ions are displaced from phosphates of the membrane lipids, destabilizing the lipopolysaccharide (LPS). This increases membrane permeability, causing cytoplasmic leaking, and killing the cell.
PDB ID 5L3G , Polymyxin .
|
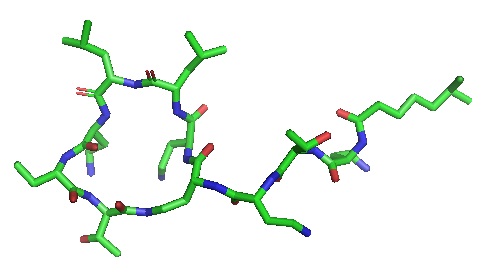
|
Conotoxins are another class of small peptide ligands (typically 10-30 amino acids) highly crosslinked by disulfide bonds. Despite their small size, these peptide ligands have very high affinities and selectivities to their cognate receptors and many of them have now become standard research tools in neuroscience.
Although cysteine bridges are quite common structural motifs in naturally occurring peptides like neurotoxins, cyclotides, somatostatin, and insulin superfamily, disulfide bridges (Figure 1) are readily reduced to their acyclic thiol form in an intracellular environment. Thus scientists have derived new methods of inducing stable conformation constraint of many peptides. For instance, advancement of organometallic chemistry has led to use of phase transfer catalyst like Grubbs catalyst in ring closing metathesis. This chemistry has been utilized by Gregory Verdine to synthesize stapled peptides which have been found with promising biological functions (Figure 2A, Table 2).
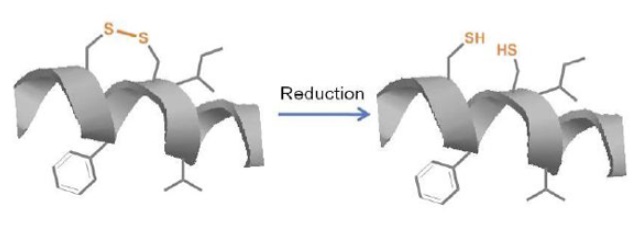
Figure 1: Scheme showing reduction of disulfide bonds in cellular environment to acyclic thiol.
In addition, availability of variety of protecting groups for amines and carboxylic acids which are cleavable under orthogonal conditions has made amide bond lactam bridges to be an alternative covalent linkage substituting disulfide bonds (Figure 2B).
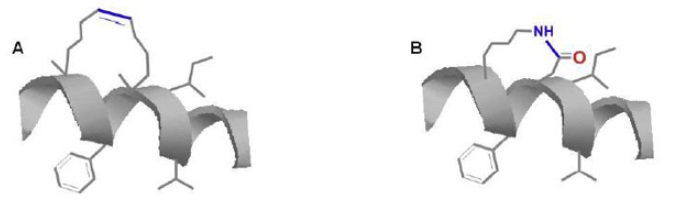
Figure 2: (A) Hydrocarbon stapled peptides synthesized through ring closing metathesis, (B) amide bond lactam bridge.
As summarized in the table 2 below, several synthetic cyclic peptides have shown desirable biological properties over their linear counterparts.
Table 2: Examples of constrained peptides with attractive biological properties.
|
Peptide
|
Type of Modification
|
Properties
|
References
|
|
α-Conotoxin
|
N- to C- terminus cyclization
|
Increased stablility in human plasma
|
Clark et al., Proc Natl Acad Sci USA 2005, 102, 13767–13772. [PNAS]
|
|
BID BH3
|
Hydrocarbon stapling using ring closing metathesis.
|
Increased protease resistance and serum stability
|
Walensky et. al., Science, 2004, 3, 1466-1470. [PMC]
|
|
NOTCH1
|
Hydrocarbon stapling using ring closing metathesis
|
Increased binding affinity towards NOTCH transactivation complex.
|
Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009 Nov 12;462(7270):182-8. doi: 10.1038/nature08543. Erratum in: Nature. 2010 Jan 21;463(7279):384 [PMC]
|
|
Glucagon-like Peptide-1
|
Side chain to side chain i, i+4 lactam bridge formation.
|
Increased receptor efficacy and enzyme stability.
|
Murage E. N. Gao G., Bisello A., Ahn J-M. J. Med. Chem. 2010,53, 6412-6420. [ACS]
|
|
DP178 (HIV35)
|
Side chain to side chain i, i+7 lactam bridge formation.
|
Increased HIV inhibitory activity as a result of stabilized a-helical conformation
|
Judice et. al., Proc. Natl. Acad. Sci. USA. 1997, 94, 13426-13430. [PNAS]
|
Reference
Pradayrol L, Jörnvall H, Mutt V, Ribet A. N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett. 1980 Jan 1;109(1):55-8. [FEBS]
Vougioukalakis GC, Grubbs RH. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem Rev. 2010 Mar 10;110(3):1746-87. [ACS]
Walensky LD, Bird GH. Hydrocarbon-stapled peptides: principles, practice, and progress. J Med Chem. 2014 Aug 14;57(15):6275-88. [PMC]
Woodward SR, Cruz LJ, Olivera BM, Hillyard DR. Constant and hypervariable regions in conotoxin propeptides. EMBO J. 1990 Apr;9(4):1015-20. [PMC]
---...---