Conjugation of antisense oligonucleotides containing bridged nucleic acids (BNAs) to palmitic acid enhances the uptake by targeted cells as well as their potency.
Prakash et al. in 2019 reported a detailed study of fatty acids conjugated to BNA modified antisense oligonucleotides containing phosphorothioate linkages. In phosphorothioate linkages (PS), a sulfur atom replaces the nonbridging oxygen, conferring resistance to digestion by nucleases. The study revealed that palmitic acid conjugated to phosphorothioate antisense oligonucleotides (PS ASOs) enhances the PS ASOs affinity to serum albumin and the potency of the ASOs in muscle tissue.
Nucleic acid-based therapeutics are promising candidates for targeting genes linked to diseases considered as undruggable. It is now well known that the conjugation of tri-antennary N-acetyl galactosamine (GalNac) to antisense oligonucleotides enhances ASO uptake into hepatocytes through asialoglycoprotein receptor (ASGR)-mediated internalization by 10- to 60-fold. The targeted receptor is a C-type lectin predominantly displayed on the plasma membranes of hepatocytes.
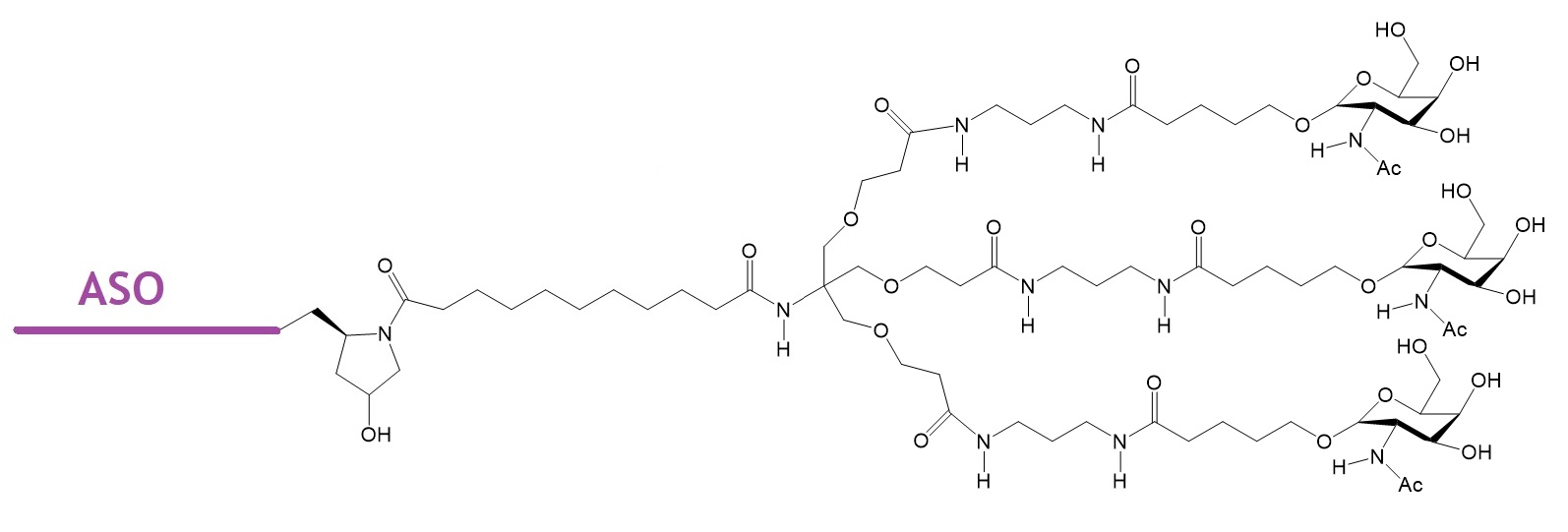
Figure 1: Example of antisense oligonucleotide conjugated to trieantennary GalNac.
As another example, a recent study revealed that conjugation of ASOs to a ligand of the glucagon-like peptide-1 receptor (GLP1R) improved uptake of the ASO by pancreatic β-cells and enhanced its potency by more than 50 fold. In this study, the GLP1R peptide antagonist eGLP1 (H(Aib)EGTFTSDVSSYLEQAAKEFIAWLVKGGPSSGAPPPSC was conjugated to the ASO modified with BNAs.
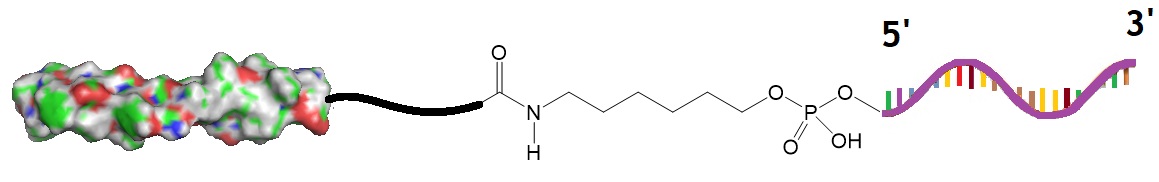
Figure 2: Antisense oligonucleotide conjugated to eGLP1.
For targeting expressed genes in muscle cells, the functional uptake of ASOs in muscle is essential for the development of therapeutics. The oxidation of long-chain fatty acids in skeletal and cardiac muscle provides the fuel needed for contractile work. In the blood, fatty acids are either complexed to albumin or covalently bound in triacylglycerols. Here they form a neutral lipid core of circulating triglyceride-rich lipoproteins such as chylomicrons or very-low-density lipoproteins. It appears that the interaction of the albumin-fatty acid complex with the endothelial membrane facilitates fatty acid uptake. The transport protein serum albumin is the most abundant plasma protein in human blood. Serum albumin is synthesized in the liver and released into the bloodstream. Albumin has several hydrophobic pockets that bind fatty acids and steroids, including a variety of drugs.
Prakash et al. investigated the affinity of ASO fatty acid conjugates to plasma proteins with the goal to enhance the uptake of ASO fatty acdi conjugates by muscle cells from the bloodstream. The study, performed in a mouse model, found that ASO conjugates containing fatty acid chains in length from 16 to 22 carbon moieties showed improved binding affinities to plasma proteins. As determined by the study, ASOs conjugated to palmitic acid improved the potency of ASOs targeting dystrophia myotonica protein kinase (DMPK), caveolin-3(Cav3), cluster of differentiation 36 (CD36), and metastasis-associated in lung adenocarcinoma transcript 1 (Malat-1) in muscle tissue.
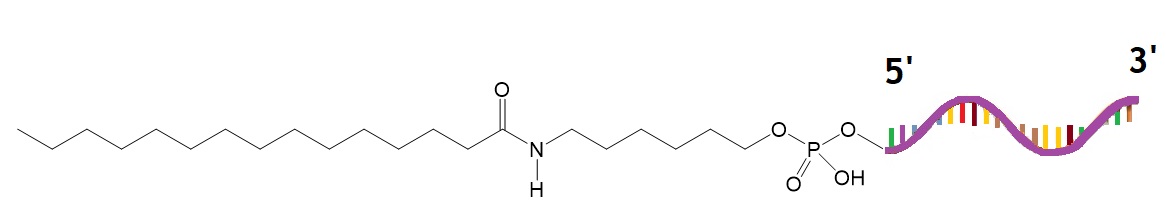
Figure 3: Antisense oligonucleotide conjugated to palmitic acid.
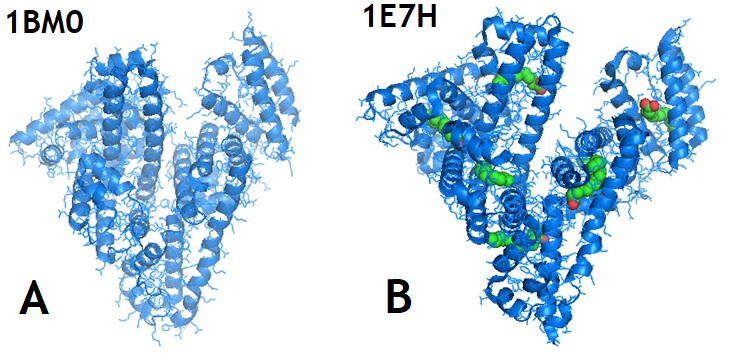
Figure 3: Structures of Human Serum Album (A; HSA PDB ID 1BM0) and HSA-palmitoyl complex (B; PDB ID 1E7H).
Human serum albumin (HSA) is an abundant plasma protein. HSA is responsible for the transport of fatty acids and also binds a wide range of drug compounds. Sugio et al. in 1999 reported the crystal structure of human serum albumin. The protein structure showed that Cys34 is the only cysteine with a free sulfhydryl group that does not participate in a disulfide linkage with any external ligand. The research group identified three tentative binding sites for long-chain fatty acids located at the surface of each protein domain. Bhattacharya et al. in 2000 solved the structure of human serum albumin in complex with palmitic acids and identified a total of 11 different fatty acid binding sites.
Reference
Ämmälä C, Drury WJ 3rd, Knerr L, Ahlstedt I, Stillemark-Billton P, Wennberg-Huldt C, Andersson EM, Valeur E, Jansson-Löfmark R, Janzén D, Sundström L, Meuller J, Claesson J, Andersson P, Johansson C, Lee RG, Prakash TP, Seth PP, Monia BP, Andersson S. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci Adv. 2018 Oct 17;4(10):eaat3386. [PMC]
A A Bhattacharya 1, T Grüne, S Curry.; Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol. 2000 Nov 10;303(5):721-32. [PubMed]
Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016 Aug 19;44(14):6518-48. [PMC].
Prakash TP, Mullick AE, Lee RG, Yu J, Yeh ST, Low A, Chappell AE, Østergaard ME, Murray S, Gaus HJ, Swayze EE, Seth PP. Fatty acid conjugation enhances potency of antisense oligonucleotides in muscle. Nucleic Acids Res. 2019 Jul 9;47(12):6029-6044. [PMC]
Roberts, T.C., Langer, R. & Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19, 673–694 (2020). [Nature]
S Sugio 1, A Kashima, S Mochizuki, M Noda, K Kobayashi. ; Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 1999 Jun;12(6):439-46. [PubMed]
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages. Bio-Synthesis also provides biotinylated mRNA as well as long circular oligonucleotides".
---...---