Like other drugs, oligonucleotide drugs will have to pass through multiple barriers before reaching their cellular targets in the human body. For oligonucleotide-based therapies to work in the central nervous system, therapeutic antisense oligonucleotides (ASOs), interfering RNAs (RNAi), or silencing RNA (siRNA), need to cross the blood-brain barrier (BBB). The term blood-brain barrier (BBB) describes the unique properties of the microvasculature of the central nervous system (See Daneman and Prat for a review).
The blood-brain barrier consists of a system of blood vessels of the central nervous system (CNS) with unique properties that allow these vessels to tightly regulate the movement of ions, molecules, and cells between the blood and the brain. The blood-brain barrier maintains brain homeostasis and prevents foreign molecules from entering the brain.
Oligonucleotide-based molecules potentially allow the design and synthesis of therapeutics for the treatment or cure of many diseases. Conjugation to hydrophobic molecules such as cholesterol and tocopherol and modifications of nucleic acids promise to enhance their delivery into cells or tissue and increase the stability of therapeutic oligonucleotides.
Most medicines approved by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and other regulatory authorities do not significally accumulate in the brain since these medicines do not cross the blood-brain barrier.
Potentially, oligonucleotide-based agents can treat or cure almost any disease and promise to be critical therapeutic drug classes of the future. Bio-conjugated oligonucleotides are now emerging from basic research and are successfully translated to the clinic.
In 2015, Nishina et al. developed a system for the delivery of short interfering RNA (siRNA) to the liver by using α-tocopherol conjugation. Nishina et al. identified a new BNA-DNA gapmer structure in which drug delivery system molecules are bound to ASOs with unlocked nucleic acid (UNA) sequences. The study found an α-tocopherol–conjugated siRNA is effective and safe for RNA interference–mediated gene silencing in vivo.
The research group extended the 5′-end of an ASO sequence by using 5′-α-tocopherol–conjugated to 4- to 7-mer UNAs for improved performance. In the liver of mice, the intravenous injection with the α-tocopherol–conjugated chimeric ASO achieved a more potent silencing than ASO alone. The UNA wing was cleaved or degraded inside the cells resulting in the release of α-tocopherol from the 13-mer gapmer ASO. This reaction resulted in activation of the gapmer. The α-tocopherol–conjugated chimeric ASO showed high efficacy, with hepatic tropism in vivo.
In 2021, Nagata et al. showed that DNA/RNA heteroduplex oligonucleotides (HDOs) conjugated to cholesterol or α-tocopherol at the 5' end of the RNA strand reach the CNS in mice and rats after subcutaneous or intravenous administration. After administration, the HDOs distribute throughout the brain, spinal cord, and peripheral tissues. The specifically selected HDOs suppressed the expression of four target genes by up to 90% in the CNS. In contrast, single-stranded ASOs conjugated to cholesterol showed limited activity. The research group observed gene knockout in major CNS cell types with the most significant effect in neurons and microglial cells. Subcutaneous delivery or dividing intravenous injections limited side effects. This study showed that cholesterol-conjugated HDOs crossed the blood-brain barrier more effectively making them candidates for future CNS drugs.
How does cholesterol help transport molecules such as oligonucleotides between cells?
Cholesterol transport occurs among organelles. Cholesterol is delivered to lysosomes by low-density plasma lipoprotein (LDL) via receptor-mediated endocytosis. Each LDL particle contains approximately 500 molecules of free cholesterol and about 1,500 molecules of esterified cholesterol. Acid lipase hydrolyzes esterified cholesterol in the lumen of the lysosome. The free cholesterol exits the lysosomal compartment to reach the plasma membrane and the endoplasmic reticulum (ER) to perform its role. The exit of cholesterol from lysosomes requires two proteins, membrane-bound Niemann-Pick C1 (NPC1) and soluble NPC2. NPC2 binds cholesterol with its iso-octyl sidechain buried and its 3ß-hydroxyl exposed.
.jpg)
Figure 1: Cholesterol, chemical structure and molecular model.
(Drugbank: DB04540)
In 2009, Kwon et al. solved the structure of the N-terminal domain of NPC1. The two proteins, NTD of NPC1, were thought to interact either with the NPC1's membrane domain or with the membrane domain of a neighboring NPC1 molecule. Several years later, Chu et al., in 2015, reported a dynamic membrane contact between peroxisomes and lysosomes. Lysosomal Synaptotagmin VII binding to the lipid PI(4,5)P2 on the peroxisomal membrane mediates the interaction. The presence of LDL-cholesterol enhances these contacts. As a result, lysosomes transport cholesterol to peroxisomes. These findings established the role of peroxisomes in intracellular cholesterol transport.
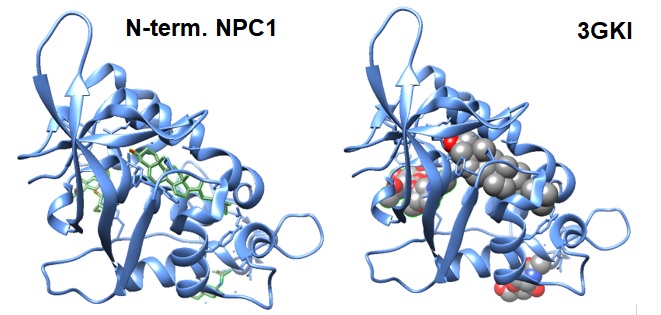
Figure 2: The structure of N-terminal domain of NPC1 shows the distinct subdomains for binding and transfer of cholesterol (3GKI. Kwon et al. 2009).
How does α-tocopherol help transport molecules such as oligonucleotides between cells?
Vitamins are essential for the growth, reproduction, and functioning of the human body. Intracellular carrier proteins solubilize and transport the fat-soluble vitamins A, D, E, and K. Vitamin E comprises a chromanol ring and an aliphatic side chain. In liver cells, the α-Tocopherol transfer protein (α-TTP) recognizes tocopherol by the three methyl groups on the chromanol ring, specifically, the methyl group at position 5, the hydroxyl group on the chromanol ring, and the orientation of the phytyl side chain. The protein α-TTP transfers α-tocopherol between membranes through direct protein-membrane interactions.
The interaction of α-TTP with phosphoinositides plays a critical role in the intracellular transport of α-tocopherol. Defects of α-TTP cause vitamin E deficiency and neurological disorders in humans.
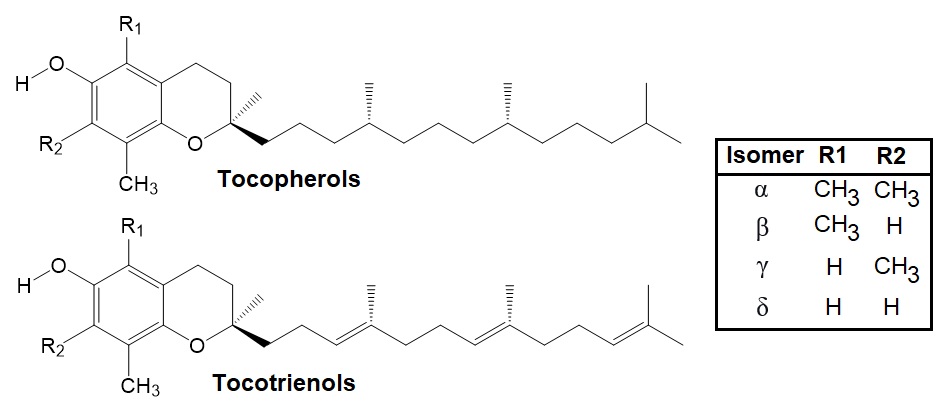
Figure 3: Vitamin E homologs. Tocopherols and tocotrienols, each have four isomers (α, β, γ and δ).
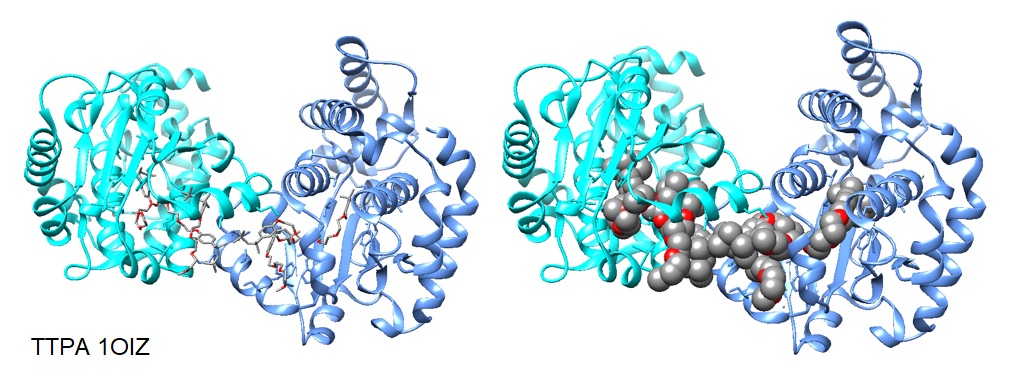
Figure 4: Structure Of Human Alpha-Tocopherol Transfer Protein [1OIZ] in complex with a fragment of Triton X-100 [PDB ID 1OIZ].
In humans, the liver protein α-Tocopherol transfer protein (α-TTP) selectively retains tocopherols and tocotrienols from dietary vitamin E. It mediates their transfer to nascent plasma very-low-density lipoprotein (VLFL). The crystal structure of alpha-TTP revealed two conformations. Tocopherol is bound such that a mobile helical surface segment hides the hydrophobic binding pocket. However, detergents are bound in an open conformation. This conformation may represent the membrane-bound form (Meier et al. 2003).
Bioconjugation chemistry is crucial for the design and synthesis of many therapeutic oligonucleotides. These molecules enable mechanistic studies at the molecular interphase of biological targets to develop agents for molecular medicine. An example for a conjugated oligonucleotide useful for photoregulatory studies is shown in figure 5.
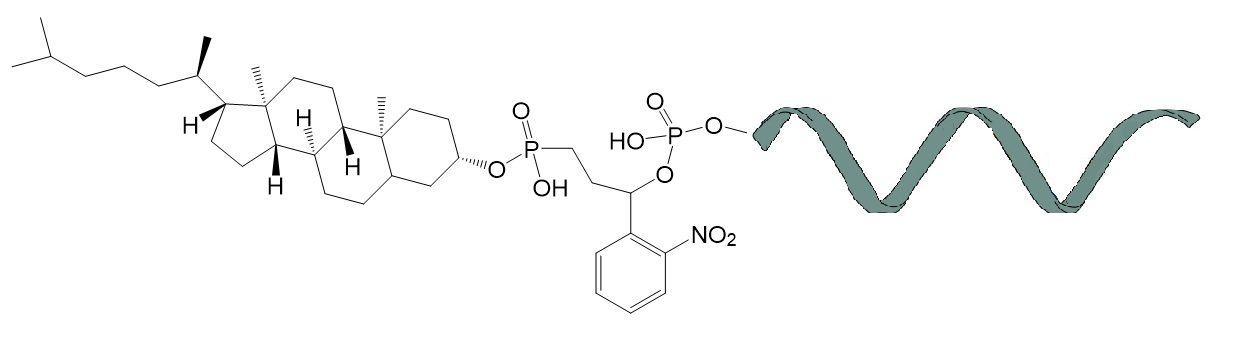
Figure 5: Example of a phtocleavable cholesterol-conjugated oligonucleotide. This molecule type can be used for the design and synthesis of caged siRNA useful for photoregulation approaches.
Reference
Benizri S, Gissot A, Martin A, Vialet B, Grinstaff MW, Barthélémy P. Bioconjugated Oligonucleotides: Recent Developments and Therapeutic Applications. Bioconjug Chem. 2019 Feb 20;30(2):366-383. [PMC]
Chu BB, Liao YC, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL, Song BL. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell. 2015 Apr 9;161(2):291-306. doi: 10.1016/j.cell.2015.02.019. Erratum in: Cell. 2021 Jan 7;184(1):289. [Cell]
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015 Jan 5;7(1):a020412. [PMC]
Kono, N. and Arai, H. (2015), Intracellular Transport of Fat-Soluble Vitamins A and E. Traffic, 16: 19-34. https://doi.org/10.1111/tra.12231
Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009 Jun 26;137(7):1213-24. [PMC]
Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. The molecular basis of vitamin E retention: structure of human alpha-tocopherol transfer protein. J Mol Biol. 2003 Aug 15;331(3):725-34. doi: 10.1016/s0022-2836(03)00724-1. PMID: 12899840.
Nagata T, Dwyer CA, Yoshida-Tanaka K, Ihara K, Ohyagi M, Kaburagi H, Miyata H, Ebihara S, Yoshioka K, Ishii T, Miyata K, Miyata K, Powers B, Igari T, Yamamoto S, Arimura N, Hirabayashi H, Uchihara T, Hara RI, Wada T, Bennett CF, Seth PP, Rigo F, Yokota T. Cholesterol-functionalized DNA/RNA heteroduplexes cross the blood-brain barrier and knock down genes in the rodent CNS. Nat Biotechnol. 2021 Aug 12. doi: 10.1038/s41587-021-00972-x. Epub ahead of print. [PubMed]
Nishina K, Piao W, Yoshida-Tanaka K, Sujino Y, Nishina T, Yamamoto T, Nitta K, Yoshioka K, Kuwahara H, Yasuhara H, Baba T, Ono F, Miyata K, Miyake K, Seth PP, Low A, Yoshida M, Bennett CF, Kataoka K, Mizusawa H, Obika S, Yokota T. DNA/RNA heteroduplex oligonucleotide for highly efficient gene silencing. Nat Commun. 2015 Aug 10;6:7969. [PMC]
Yang, J., Chen, C., and Tang, X. (2018) Cholesterol-modified caged siRNA for photoregulating exogenous gene expression. Bioconjug. Chem. 29, 1010-1015. [ACS]
---...---
Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---