Phosphoryl guanidine oligo-2’-O-methylribonucleotides (2’-OMe PGOs) are a newer version of uncharged or neutral RNA analogs with high RNA affinity. 2’-OMe PGOs penetrate through bacterial cell walls more efficiently.
Neutral oligonucleotides are short sequences of nucleotides that do not carry any net positive or negative charge. In their natural form, nucleic acids have a negative charge due to phosphate groups in their backbone. However, certain modifications of the nucleotide structure allow removing the backbone charge. These DNA or RNA oligonucleotides typically contain neutral functional groups at their inter-nucleoside linkages.
One common modification used in therapeutic oligonucleotides is the phosphorothioate linkage. In this modification, a sulfur atom replaces one of the non-bridging oxygen atoms in the phosphate group. This substitution renders the internucleotide linkage resistant to nuclease degradation but does not totally remove the charges carried by phosphate groups. For therapeutic uses, phosphorothioate and phosphorodithioate modifications are often chemically introduced into synthetic nucleic acids. The enhanced affinity of racemic phosphorothioate DNA with target oligonucleotides arises from diastereomer-specific hydrogen bonds and hydrophobic contacts.
Oligonucleotides with neutral backbones have now found applications in various research and therapeutic applications. Examples are their use as control sequences in experiments studying the effects of charges on nucleic acid interactions or to determine the specific contribution of the charged backbone to a biological process.
Two types of nucleic analogs that render the backbone of oligonucleotides neutral are peptide nucleic acids (PNAs) and phosphorodiamidate morpholino oligomers (PMO). Both types have been well studied over the past 20 years and can sequence-specifically bind natural DNA and RNA. To find better therapeutic solutions, the search for oligonucleotide therapeutics able to efficiently penetrate cells without transfection agents continues to this day.
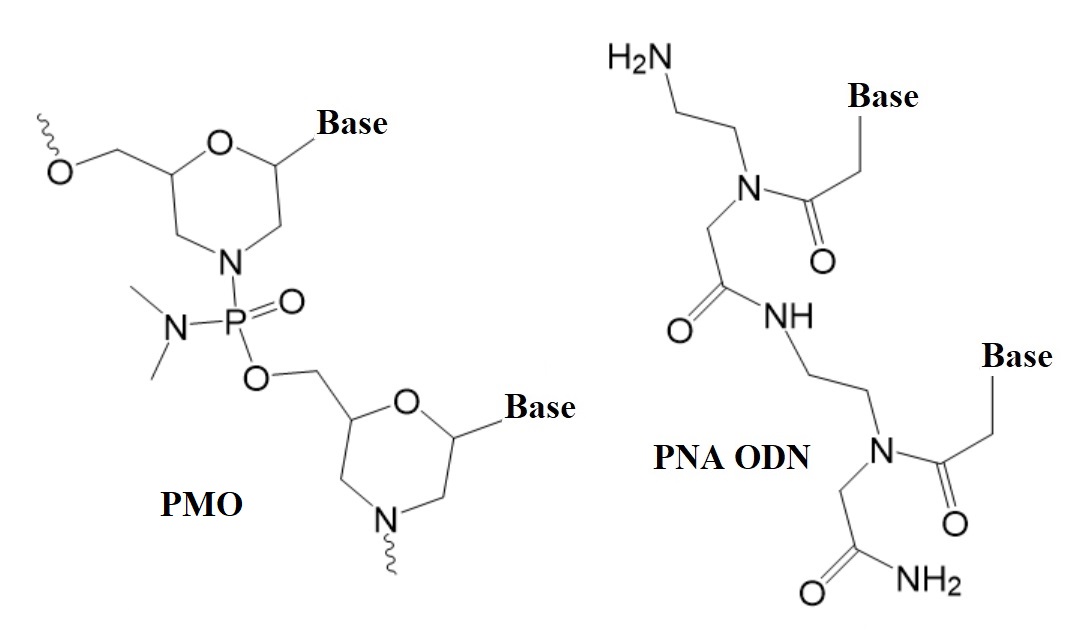
Figure 1: Structures of neutral nucleic acid analogues used in therapeutic oligonucleotides (ODNs).
PMO: Phosphorodiamidate morpholino oligonucleotides. PNA ODN: Peptide nucleic acid oligonucleotides.
{ Morpholinos or PMOs; PNAs }
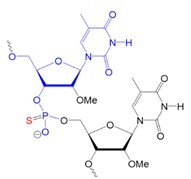
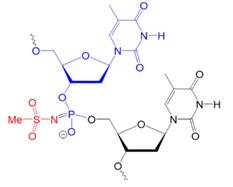
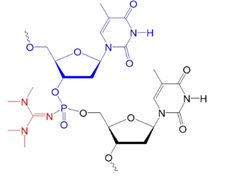
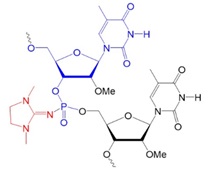
Recently, scientists investigated new types of neutral oligonucleotides for their use in therapeutic applications, such as antisense oligonucleotide-based drugs, where the neutral charge can help improve their stability, biodistribution, and cellular uptake. Figure 2 shows the structures for phosphorothioates oligodeoxynucleotides (PS ODNs), mesyl phosphoramidate oligodeoxynucleotides (μ-ODNBs), tetramethyl phosphoryl guanidine oligonucleotides (2’-OMe PGO ODNs), and phosphoryl guanidine oligo-2′-O-methylribonucleotides (2’-OMe PGO ODN).
|
PS ODN:
Oligodeoxynucleotide
phosphorothioate
|
μ-ODNB:
oligodeoxynucleotide
mesyl phosphoramidate
|
TMG ODN:
tetramethyl phosphoryl guanidine
|
2’-OMe PGO ODN: phosphoryl guanidine oligo-2′-O-methylribonucleotide
|
|
|
|
|
|
|
ASOs, siRNAs,
therapeutic oligonucleotides, and others.
|
Su et al. 2019.
Parallel G-quadruplexes.
|
Kupryushkin et al. 2014.
Pavlova et al. 2021.
Sue t al. 2019.
ASOs, siRNAs,
Parallel G-quadruplexes.
|
Su et al. 2019.
Pavlova et al. 2021.
ASOs, siRNAs,
Parallel G-quadruplexes.
|
Figure 2: Structures of nucleic acid analogs used in oligonucleotides (ODNs) containing inter-nucleoside modifications.
PS ODN: Oligodeoxynucleotide phosphorothioate or PS oligodeoxynucleotide, negatively charged.
μ-ODNB: Oligodeoxynucleotide mesyl phosphoramidate, negatively charged.
TMG ODN: Oligonucleotide tetramethyl phosphoryl guanidine, neutral.
2’-OMe PGO ODN: Oligonucleotide phosphoryl guanidine oligo-2′-O-methylribonucleotide, neutral.
In 2014, Kupryushkin et al. reported the synthesis of a new neutral nucleic acid analog containing a phosphoryl guanidine group. The oxidation of a polymer-supported dinucleotide 2-cyanoethyl phosphite by iodine in the presence of 1,1,3,3-tetramethyl guanidine yielded a dinucleotide with an internucleoside tetramethyl phosphoryl guanidine (Tmg) group as the main product. The Tmg group is stable under conditions of solid-phase DNA synthesis and subsequent cleavage and deprotection with ammonia.
The research group showed that the Tmg functional group has hydrophobic properties characterized by a longer retention time (τR) of the Tmg group-containing oligonucleotides compared with that of the unmodified oligonucleotide. Several modified oligothymidylates up to 20 nucleotides long have been synthesized, with one or two Tmg groups at various positions in the oligonucleotide chain. MALDI- TOF mass spectrometry allows confirmation of the presence of tetramethyl phosphoryl guanidine groups in the synthetic oligonucleotides. All oligonucleotides studied having one or more Tmg groups bind their complementary DNA or RNA with affinities similar to natural oligodeoxyribonucleotides. Further, Kupryushkin et al. pointed out that, unlike the oligonucleotide analogs PNA or PMO, phosphoryl guanidine derivatives can be synthesized by conventional phosphoramidite chemistry using a standard DNA synthesizer.
In 2018, Pavlova et al. showed that SDS-PAGE is suitable for the analysis of the un-charged oligonuclotides such as morpholino oligonucleotides (PMOs) and peptide nucleic acids (PNAs). The research group demonstrated that sodium dodecyl sulfate (SDS) establish hydrophobic interactions with these nucleic acids. SDS adds a net negative charge to the polymers making these molecules mobile in polyacrylamide slab gels under the influence of an electric field.
In 2019, Su et al. evaluated structural, thermodynamic, and kinetic properties of the parallel G-quadruplexes formed by oligodeoxynucleotides d(G4T), d(TG4T), and d(TG5T), in which all phosphates were replaced with N-methanesulfonyl (mesyl) phosphoramidate or phosphoryl guanidine groups resulting in either negatively charged or neutral DNA sequences, respectively. The research study established that all modified sequences formed G-quadruplexes of parallel topology. The presence of modifications caused a decrease in thermal stability relative to unmodified G4s.
Compared to negatively charged G4s, the assembly of neutral G4 DNA species was faster in the presence of sodium ions than potassium ions but was independent of the salt concentration used. The formation of mixed G4s composed of both native and neutral G-rich strands was confirmed using native gel electrophoresis, size-exclusion chromatography, and ESI-MS. The study showed that nucleic acids modified with N-methanesulfonyl (mesyl) phosphoramidate or phosphoryl guanidine groups are compatible with G-quadruplex formation.
Also in 2019, Lomzov et al. reported the physicochemical properties of diastereomers of a mono-substituted phosphoryl guanidine trideoxyribonucleotide d(TpCp*A), including information on isolation, identification, treatment with snake venom phosphodiesterase, structural analysis by 1D and 2D NMR spectroscopy as well as restrained molecular dynamics analysis.
Also in 2019, Skvortsova et al. reported that phosphoryl guanidine oligo-2′-O-methylribonucleotides (2′-OMe PGOs specific for the alanine dehydrogenase-encoding ald gene inhibited the growth of Mycobacterium smegmatis and downregulated ald expression at both the transcriptional and translational levels through an RNase H-independent mechanism with a higher biological activity than its phosphorothioate oligonucleotide. The observed antisense activity and efficient uptake of the new RNA analog, 2′-OMe PGO, by intracellular microorganisms could promote the development of novel therapeutic strategies to treat tuberculosis and prevent the emergence of drug-resistant mycobacterial strains.
In 2021, Pavlova et al. investigated silencing effects in cell cultures of siRNAs modified with phosphoryl guanidine (PGs) groups. PG modifications make oligonucleotides resistant to snake venom phosphodiesterase.
Adding the PG group to siRNAs decreased their thermodynamic stability but resulted in increased resistance to RNase A. A gene silencing experiment showed that the PG-modified siRNA retained activity if the passenger strand contained PG modifications. However, the PG group introduces steric hindrances limiting access of nucleases to neighboring sites. Modifying the guide strand with PG groups abrogates the silencing effect. Pavlova et al. suggested that adding nucleic acids containing the PG group to the passenger strand could eliminate off-target effects due to the passenger strand unintentionally entering RISC.
In 2022, Dyudeeva and Pyshnaya demonstrated that PGOs could act as primers in the presence of a fragment of human ribosomal RNA with a complex spatial structure. This study showed that the proportion (in %) of abortive elongation products of a PGO primer depends on the ionic strength of the buffer, the nucleotide sequence of the primer, and the presence and location of phosphoryl guanidine groups in the primer. The results indicate the suitability of PGOs as primers for reverse-transcription PCR.
Reference
Antisense Oligonucleotides in Alzheimer’s Research [ R&D World ]
Dyudeeva ES, Pyshnaya IA. Phosphoryl guanidine oligonucleotides as primers for RNA-dependent DNA synthesis using murine leukemia virus reverse transcriptase. Vavilovskii Zhurnal Genet Selektsii. 2022 Feb;26(1):5-13. [PMC]
Kupryushkin MS, Pyshnyi DV, Stetsenko DA. Phosphoryl guanidines: a new type of nucleic Acid analogues. Acta Naturae. 2014 Oct;6(4):116-8. [PMC]
Lomzov AA, Kupryushkin MS, Shernyukov AV, Nekrasov MD, Dovydenko IS, Stetsenko DA, Pyshnyi DV. Data for isolation and properties analysis of diastereomers of a mono-substituted phosphoryl guanidine trideoxyribonucleotide. Data Brief. 2019 Jun 20;25:104148. [PMC]
Pavlova AS, Dyudeeva ES, Kupryushkin MS, Amirkhanov NV, Pyshnyi DV, Pyshnaya IA. SDS-PAGE procedure: Application for characterization of new entirely uncharged nucleic acids analogs. Electrophoresis. 2018 Feb;39(4):670-674. [Wiley]
Pavlova AS, Yakovleva KI, Epanchitseva AV, Kupryushkin MS, Pyshnaya IA, Pyshnyi DV, Ryabchikova EI, Dovydenko IS. An Influence of Modification with Phosphoryl Guanidine Combined with a 2'-O-Methyl or 2'-Fluoro Group on the Small-Interfering-RNA Effect. Int J Mol Sci. 2021 Sep 10;22(18):9784. [PMC]
Skvortsova YV, Salina EG, Burakova EA, Bychenko OS, Stetsenko DA, Azhikina TL. A New Antisense Phosphoryl Guanidine Oligo-2'-O-Methylribonucleotide Penetrates Into Intracellular Mycobacteria and Suppresses Target Gene Expression. Front Pharmacol. 2019 Sep 19;10:1049. [PMC]
Su Y, Fujii H, Burakova EA, Chelobanov BP, Fujii M, Stetsenko DA, Filichev VV. Neutral and Negatively Charged Phosphate Modifications Altering Thermal Stability, Kinetics of Formation and Monovalent Ion Dependence of DNA G-Quadruplexes. Chem Asian J. 2019 Apr 15;14(8):1212-1220. [Pubmed]
Yamasaki K, Akutsu Y, Yamasaki T, Miyagishi M, Kubota T. Enhanced affinity of racemic phosphorothioate DNA with transcription factor SATB1 arising from diastereomer-specific hydrogen bonds and hydrophobic contacts. Nucleic Acids Res. 2020 May 7;48(8):4551-4561. [PMC]
---...---
Bio-Synthesis provides a full spectrum of bio-conjugation services including high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---