Antisense oligonucleotides (ASOs) are short, synthetic strands of DNA or RNA that bind to specific RNA targets and modulate gene expression. ASOs can inhibit gene expression, promote gene expression, or alter gene splicing.
Mechanism of Action
There are two main mechanisms of action for ASOs:
[1] RNase H-dependent degradation
The enzyme RNase H degrades RNA when hybridized to DNA. ASOs complementary to a target RNA can bind to it and form a hybrid duplex. RNase H then recognizes the duplex and degrades the RNA.
[2] Steric blocking
Steric blocking ASOs bind to targeted RNA, such as mRNA, and prevent it from interacting with other molecules, for example, with ribosomes or splicing factors. ASOs can block the translation of the RNA into a protein or alter RNA splicing.
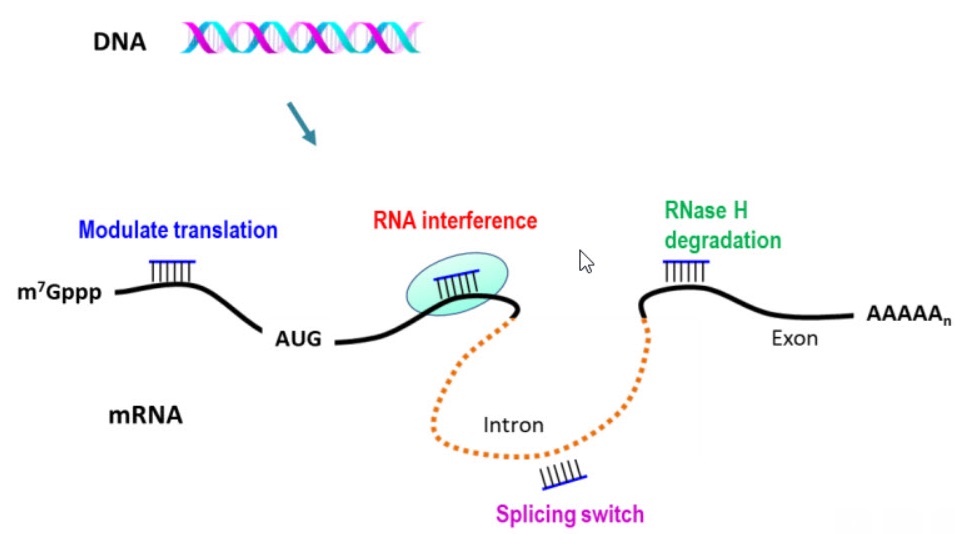
Applications of ASOs
ASOs potentially allow the development of therapeutics for a wide range of diseases, including:
Genetic disorders: ASOs allow targeting and silencing mutant genes that cause genetic disorders, such as Duchenne muscular dystrophy and spinal muscular atrophy.
Infectious diseases: ASOs enable the targeting and silencing of viral RNAs, such as those of SARS-CoV-2 (COVID-19), HIV, and hepatitis C virus.
Cancer: Well-designed ASOs targeting oncogenes that promote cancer growth and progression silencing these genes.
Cardiovascular diseases: ASOs allow the targeting and silencing of disease-causing genes, including cardiovascular diseases, atherosclerosis, or heart failure.
Neurodegenerative diseases: ASOs enable the development of oligonucleotide-based therapeutics to treat neurodegenerative diseases including Alzheimer's disease and Huntington's disease.
Advantages of ASOs
ASOs appear to have several advantages over other gene therapy approaches:
ASOs are highly specific and allow targeting of any RNA sequence.
ASOs are relatively easy to synthesize and deliver to cells.
ASOs exhibit an excellent safety profile and are generally well-tolerated.
Challenges to Solve
However, ASOs are still under development. Before the use of ASOs in clinical practice, several challenges will need to be overcome. ASOs are unstable in the bloodstream and are readily degraded by enzymes. Also, to be effective, delivery to the target cells is essential.
Researchers are in progress to develop new ASO chemistries and delivery methods to overcome these challenges. For example, researchers have developed ASOs that are resistant to degradation and that can be conjugated to carrier molecules for delivery into specific cells.
Therapeutic Antisense Oligonucleotides
Antisense oligonucleotide (ASO) therapeutics utilize short, synthetic DNA or RNA oligonucleotides to modulate gene expression for gene therapies. ASOs bind to specific RNA targets and either inhibit or promote gene expression or alter RNA splicing.
ASO therapeutics are relatively easy to synthesize, very specific, and have a good safety profile. The medical community investigates ASOs for a wide range of diseases, including genetic disorders, infectious diseases, cancer, cardiovascular diseases, and neurodegenerative diseases.
Examples of ASO therapeutics that are currently approved or in clinical trials are:
Spinal muscular atrophy (SMA): Nusinersen (Spinraza) is an ASO approved to treat SMA, a genetic disorder that causes motor neuron loss and muscle weakness. Nusinersen works by increasing the production of a protein called SMN, which is essential for motor neuron health.
Duchenne muscular dystrophy (DMD): Eteplirsen (Exondys 51) and Viltolarsen (Viltepso) are approved to treat DMD, which causes progressive muscle weakness and death. Eteplirsen and Viltolarsen promote the skipping of a specific exon in the dystrophin gene, allowing the production of a functional dystrophin protein.
Transthyretin amyloidosis (ATTR): Inotersen (Tegsedi) and Patisiran (Onpattro) are approved to treat ATTR. This rare genetic disorder causes amyloid deposits to form in various tissues throughout the body. Inotersen and Patisiran silence the transthyretin gene, which leads to a reduction in the production of transthyretin protein.
Hepatitis B virus (HBV): Givosiran (Givlaari) is approved to treat chronic HBV infection in adults with compensated liver disease. Givosiran silences the HBV gene, reducing the production of the HBV virus.
COVID-19: Other ASOs are currently in clinical trials to treat COVID-19. These ASOs target the SARS-CoV-2 virus, which causes COVID-19, and prevent it from replicating.
The rapidly developing field of ASO therapeutics may enable the treatment of many diseases.
Review Articles
Adachi H, Hengesbach M, Yu YT, Morais P. From Antisense RNA to RNA Modification: Therapeutic Potential of RNA-Based Technologies. Biomedicines. 2021 May 14;9(5):550. [PMC]
Anderson BA, Freestone GC, Low A, De-Hoyos CL, Iii WJD, Østergaard ME, Migawa MT, Fazio M, Wan WB, Berdeja A, Scandalis E, Burel SA, Vickers TA, Crooke ST, Swayze EE, Liang X, Seth PP. Towards next generation antisense oligonucleotides: mesylphosphoramidate modification improves therapeutic index and duration of effect of gapmer antisense oligonucleotides. Nucleic Acids Res. 2021 Sep 20;49(16):9026-9041. [PMC]
Anthony K. RNA-based therapeutics for neurological diseases. RNA Biol. 2022;19(1):176-190. doi: 10.1080/15476286.2021.2021650. [PMC]
Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259-93. [Annual Reviews]
Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-Targeted Therapeutics. Cell Metab. 2018 Apr 3;27(4):714-739. doi: 10.1016/j.cmet.2018.03.004. Erratum in: Cell Metab. 2019 Feb 5;29(2):501. [Cell-Metabolism]
Crooke ST, Liang XH, Baker BF, Crooke RM. Antisense technology: A review. J Biol Chem. 2021 Jan-Jun;296:100416. doi: 10.1016/j.jbc.2021.100416. Epub 2021 Feb 16. PMID: 33600796; PMCID: PMC8005817. [PMC]
Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The Limitless Future of RNA Therapeutics. Front Bioeng Biotechnol. 2021 Mar 18;9:628137. [PMC]
Deleavey GF, Damha MJ. Designing chemically modified oligonucleotides for targeted gene silencing. Chem Biol. 2012 Aug 24;19(8):937-54. [Sciencedirect]
Egli M, Manoharan M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 2023 Apr 11;51(6):2529-2573. [PMC]
Gagliardi M, Ashizawa AT. The Challenges and Strategies of Antisense Oligonucleotide Drug Delivery. Biomedicines. 2021 Apr 16;9(4):433. [PMC]
Gait MJ, Agrawal S. Introduction and History of the Chemistry of Nucleic Acids Therapeutics. Methods Mol Biol. 2022;2434:3-31. [PMC]
Goodchild, J.; 2011. Therapeutic Oligonucleotides, Methods and Protocols. MIMB, volume 764. [Springer Book]
Halloy F, Iyer PS, Ćwiek P, Ghidini A, Barman-Aksözen J, Wildner-Verhey van Wijk N, Theocharides APA, Minder EI, Schneider-Yin X, Schümperli D, Hall J. Delivery of oligonucleotides to bone marrow to modulate ferrochelatase splicing in a mouse model of erythropoietic protoporphyria. Nucleic Acids Res. 2020 May 21;48(9):4658-4671. [PMC]
Hirabayashi Y, Maki K, Kinoshita K, Nakazawa T, Obika S, Naota M, Watanabe K, Suzuki M, Arato T, Fujisaka A, Fueki O, Ito K, Onodera H. Considerations of the Japanese Research Working Group for the ICH S6 & Related Issues Regarding Nonclinical Safety Assessments of Oligonucleotide Therapeutics: Comparison with Those of Biopharmaceuticals. Nucleic Acid Ther. 2021 Apr;31(2):114-125. [PMC]
Järver P, O'Donovan L, Gait MJ. A chemical view of oligonucleotides for exon skipping and related drug applications. Nucleic Acid Ther. 2014 Feb;24(1):37-47. [PMC]
Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016 Aug 19;44(14):6518-48. [PMC]
Ji D, Feng H, Liew SW, Kwok CK. Modified nucleic acid aptamers: development, characterization, and biological applications. Trends Biotechnol. 2023 Nov;41(11):1360-1384. [cell]
Kilanowska A, Studzińska S. In vivo and in vitro studies of antisense oligonucleotides - a review. RSC Adv. 2020 Sep 17;10(57):34501-34516. [PMC]
Kilikevicius A, Meister G, Corey DR. Reexamining assumptions about miRNA-guided gene silencing. Nucleic Acids Res. 2022 Jan 25;50(2):617-634. [PMC]
Matsuo M. Antisense Oligonucleotide-Mediated Exon-skipping Therapies: Precision Medicine Spreading from Duchenne Muscular Dystrophy. JMA J. 2021 Jul 15;4(3):232-240. [PMC]
Moumné L, Marie AC, Crouvezier N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics. 2022 Jan 22;14(2):260. [PMC]
Ramsden D, Belair DG, Agarwal S, Andersson P, Humphreys S, Dalmas DA, Stahl SH, Maclauchlin C, Cichocki JA. Leveraging microphysiological systems to address challenges encountered during development of oligonucleotide therapeutics. ALTEX. 2022;39(2):273–296. [Altex]
Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020 Oct;19(10):673-694. [PMC]
Sasaki T, Hirakawa Y, Yamairi F, Kurita T, Murahashi K, Nishimura H, Iwazaki N, Yasuhara H, Tateoka T, Ohta T, Obika S, Kotera J. Altered Biodistribution and Hepatic Safety Profile of a Gapmer Antisense Oligonucleotide Bearing Guanidine-Bridged Nucleic Acids. Nucleic Acid Ther. 2022 Jun;32(3):177-184. [PubMed]
Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018 Feb 28;46(4):1584-1600. [PubMed]
Tarn WY, Cheng Y, Ko SH, Huang LM. Antisense Oligonucleotide-Based Therapy of Viral Infections. Pharmaceutics. 2021 Nov 26;13(12):2015. [PMC]
Thakur S, Sinhari A, Jain P, Jadhav HR. A perspective on oligonucleotide therapy: Approaches to patient customization. Front Pharmacol. 2022 Oct 19;13:1006304. [PMC]
Xiong H, Veedu RN, Diermeier SD. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int J Mol Sci. 2021 Mar 24;22(7):3295. [PMC]
Other links
Backbone modifications: https://www.biosyn.com/faq/What-are-Backbone-Modifications-in-Oligonucleotide-Synthesis.aspx
Duplex Stability: https://www.biosyn.com/tew/oligo-modifications-for-increased-duplex-stability-and-nuclease-resistance.aspx
Epigenetic Modifications: https://www.biosyn.com/tew/oligonucleotides-containing-epigenetic-cytosine-modifications.aspx
https://www.biosyn.com/rna-modifications.aspx#!
Metabolic stable siRNA: https://www.biosyn.com/tew/Argonaute-2-can-bind-metabolic-stable-siRNAs.aspx#!
Modifications to avoid off-target effects: https://www.biosyn.com/tew/Off-Target-Effects-in-small-interfering-RNA-or-siRNA.aspx#!
Nucleotide analogs: https://www.biosyn.com/tew/Therapeutic-nucleoside-and-nucleotide-analogs.aspx#!
Synthetic RNA mimics: https://www.biosyn.com/tew/2%E2%80%99-O-NMA-phosphoramidites-enable-the-synthesis-of-RNA-mimics-useful-for-antisense-therapeutics.aspx#!
Targeted delivery of siRNA: https://www.biosyn.com/tew/Targeted-delivery-of-siRNA.aspx#!
Common Chemical Modifications Used In Synthetic RNAs
|
First Generation
Modified phosphate backbone resulting in greater resistance to nucleases.
Phosphorothioate
|
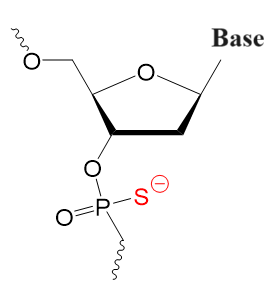
PS
|
|
Second Generation
2’-Substituted resulting in tighter target binding.
2’-Fluoro-phosphorothioate,
2’-O-Methyl-phosphorothioate (OMe PS),
2’-O-Methyl-O-ethyl-phosphorothioate (O-MOE PS).
|
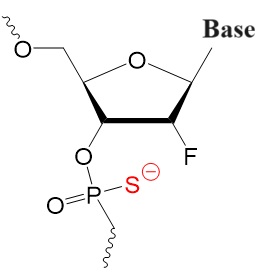 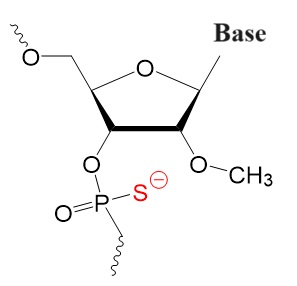 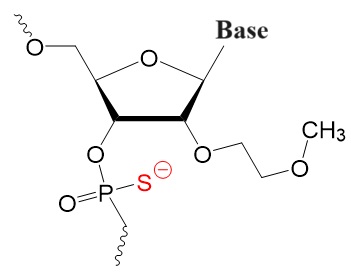
2'-F PS 2'-OMe PS 2'-O-OME PS
|
|
Third Generation
Non-ribose with altered charge and flexibility.
Peptide Nucleic Oligomers (PNAs or PNOs),
Bridged Nucleic Acids (BNAs),
Locked Nucleic Acids (LNAs),
Phosphoramidite Morpholino Oligomers (PMOs).
|
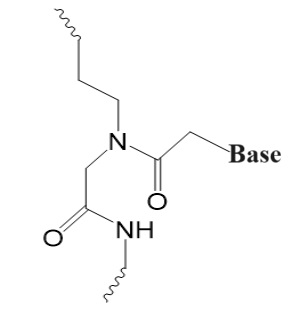 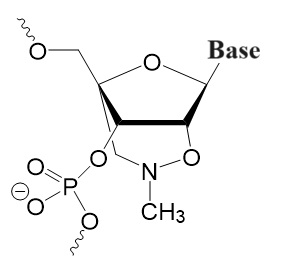 .jpg) 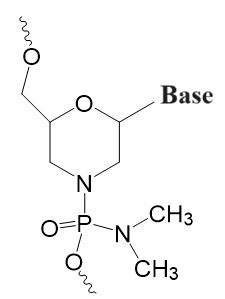
PNA/PNO BNA LNA PMO
|
---...---