RNA molecules like messenger RNA are known to have modifications on their 5’-terminal ends. In recent years, scientists have confirmed many 5’-modified RNA molecules, including RNA modified with adenosine-containing cofactors from the B vitamin group, found in all kingdoms of life.
Thiamine adenosine triphosphate (ThATP) is a vitamin B1 derivative that accumulates in Escherichia coli and other organisms in response to metabolic stress conditions. Thiamine and its phosphorylated derivatives (mono-, di-, and triphosphate) occur naturally in most cells. Frédérich et al., in 2009, reported the presence of the thiamine derivative, adenosine thiamine triphosphate, in Escherichia coli as a response to carbon starvation.
To enable the discovery of 5’-thiamine-capped RNA in organisms, Möhler et al. in 2020 synthesized thiamine adenosine dinucleotides and 5’-thiamine-capped RNA utilizing phosphorimidazolide chemistry. The research group showed that T7 RNA polymerase in the presence of divalent magnesium cations caps RNA with thiamine-ATP and thiamine adenosine diphosphate (thiamine-ADP). This approach allows the capping of 5′-triphosphate RNAs, independent of length, structure, or nucleotide composition, after conversion to the 5′-monophosphate by, e.g., polyphosphatases. In vitro transcription allowed the synthesis of larger capped RNA molecules.
Also, Möhler et al. modified transcripts containing the thiamine modification with biotin, allowing the separation of in vitro transcribed 5’-thiamine RNA from 5’-triphosphate RNA. This new method now provides access to 5’-thiamine-capped RNA useful for developing thiamine-specific RNA capture protocols for discovering and confirming 5’-thiamine-capped RNAs in different organisms.
Vitamin B1 is an essential micronutrient the human body cannot make but is found in food, commercially synthesized, and used in supplements and medications. Metabolic reactions in mammals and humans require phosphorylated forms, such as the metabolization of glucose and amino acids. Five natural thiamine phosphates are known: thiamine monophosphate (ThMP), thiamine pyrophosphate (TPP), thiamine triphosphate (ThTP), adenosine thiamine diphosphate (AThDP), and adenosine thiamine triphosphate (AThTP).Vitamin B1 is known as thiamin. The thiamin anion binds protons in a cooperative process. Figure 1 shows the reaction scheme for the cooperative binding of protons by the thiamin anion.
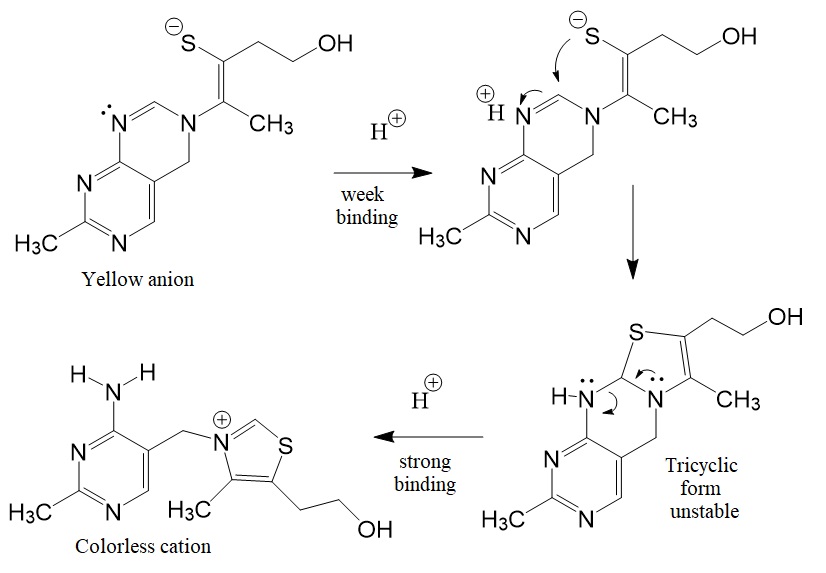
Figure 1: Reaction scheme for the cooperative binding of protons by the thiamine anion (David Metzler. Biochemistry. 2nd Edition).
Thiamine (vitamin B1) functions as a coenzyme, for example, during the fermentation of sugar to ethanol by yeast. Bacteria, fungi, and plants synthesize thiamine from 1-deoxyxylulose 5-phosphate. 1-Deoxyxylose 5-phosphate is an intermediate in the nonmevalonate pathway of polyprenyl synthesis. David Metzler reported that Mizuhare, in 1950, discovered that the hydrogen atom in the two position of the thiazolium ring, between the sulfur and the nitrogen atoms, exchanged quickly with deuterium of 2H2O. The pKa of this proton has been estimated as ~ 18, low enough to permit rapid dissociation and replacement with 2H.
In recent decades, scientists have determined the structures for many thiamine diphosphate-dependent enzymes, including bacterial pyruvate oxidases, yeast, pyruvate decarboxylases, transketolase, and benzoyl formate decarboxylases. The different enzymes have different sequences and catalyze different reactions. However, the thiamin diphosphate is bound similarly in all of them. Figure 2 shows the crystal structure of the thiamine diphosphate-dependent enzyme pyruvate decarboxylase from yeasts.
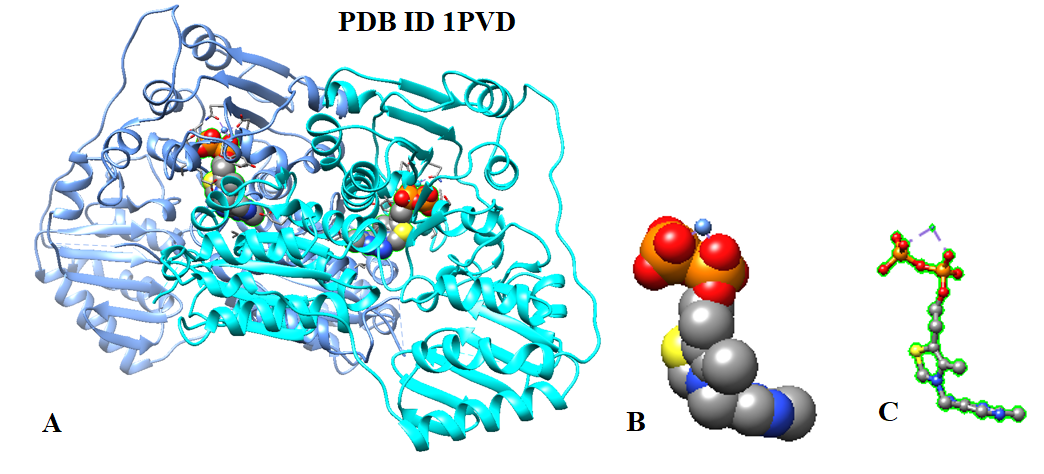
Figure 2: A; Crystal structure of the thiamine diphosphate dependent enzyme pyruvate decarboxylase from the yeast saccharomyces cerevisiae at 2.3 Angstroms resolution [PDB ID 1PVD]. B and C; Structural models of thiamine diphosphate.
Reference
Arjunan P, Umland T, Dyda F, Swaminathan S, Furey W, Sax M, Farrenkopf B, Gao Y, Zhang D, Jordan F. Crystal structure of the thiamin diphosphate-dependent enzyme pyruvate decarboxylase from the yeast Saccharomyces cerevisiae at 2.3 A resolution. J Mol Biol. 1996 Mar 1;256 (3):590-600. [PubMed]
Frédérich M, Delvaux D, Gigliobianco T, Gangolf M, Dive G, Mazzucchelli G, Elias B, De Pauw E, Angenot L, Wins P, Bettendorff L (2009). Thiaminylated adenine nucleotides. Chemical synthesis, structural characterization and natural occurrence. The FEBS journal 276, 3256-3268. [FEBS]
Metzler, David. Biochemistry. The chemical reactions of living cells. 2nd Edition 2003 [ELSEVIER]
Möhler M, Höfer K, Jäschke A. Synthesis of 5'-Thiamine-Capped RNA. Molecules. 2020 Nov 24;25(23):5492. [PMC]
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated and capped mRNA as well as long circular oligonucleotides".
---...---