The post-transcriptional mechanism RNA interference silences gene expression by cleaving long double stranded RNA (dsRNA) into short dsRNA fragments, which are then unwound into single stranded RNAs to hybridize to target mRNA for degradation via the silencing complex (Agrawal et al., 2003; Li, 2008; Wilson et al, 2013). RNA interference provides a unique opportunity to suppress gene expression without permanently inactivating the gene or introducing mutation (by integrating into the genomic DNA). The ability of siRNAs to suppress gene expression by repeatedly targeting mRNAs for degradation constitutes a distinct pharmacological advantage.
Several notable advances have been made in the last decade. In 2010, a phase I clinical trial showed that systemically delivered self-assembled nanoparticle containing siRNA targeting ribonucleotide reductase induced RNA interference in the tumor tissue of melanoma patients (Davis et al, 2010). In 2013, the results from a Phase I clinical trial of ALN-VSP, a lipid nanoparticle carrying double siRNAs targeting vascular endothelial growth factor A (VEGF-A) and kinesin spindle protein KSP, in cancer patients was reported (Tabernero et al., 2013).
In 2018, Onpattro was the first siRNA based drug developed by Alnylam, Inc. to be approved by Food & Drug Administration (FDA) for treating polyneuropathy. It targets the mRNA encoding transthyretin (TTR), which transports thyroid hormone and retinol binding protein.
Acute hepatic porphyria is a rare inherited disorder that causes short lasting but rapid onset of life-threatening symptoms such as severe abdominal pain, high blood pressure, fever, neuropathic pain, etc. It is caused by abnormal buildup of porphyrin, which forms the heme group of hemoglobin found in red blood cells to transport oxygen, in nervous system or skin. Heme is also essential for the function of catalase, peroxidase, p450 liver cytochromes, etc. The disorder could result from deficiency (i.e. genetic mutation is inherited) in any of the enzymes in the heme biosynthesis pathway, ex. acute intermittent porphyria, variegate porphyria, hereditary coproporphyria. However, the symptoms are not caused by the reduction in heme (as it can be compensated by other enzymes) but rather due to the toxicity associated with the high level of porphyrins. For instance, the acute attack may occur when aminolaevulinic acid synthase is induced by alcohol, reduced carbohydrate intake, etc.
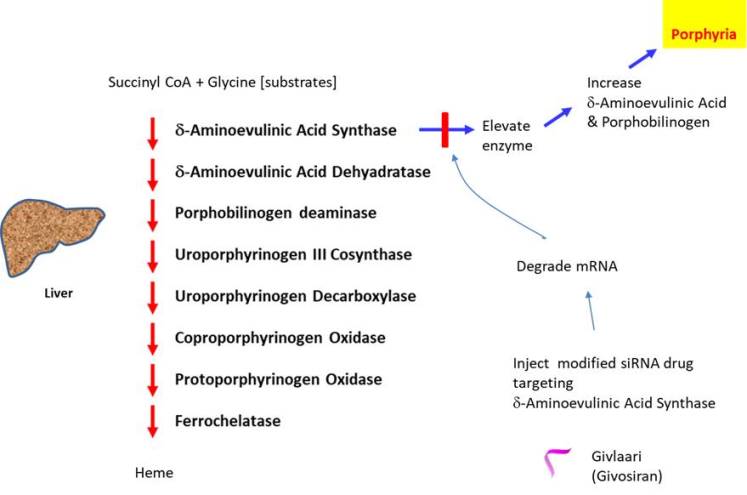
In 2019, Givlaari was the second siRNA based drug to be approved by FDA (also called Givosiran: approved by European Medicines Agency in 2020), which was developed by Alnylam Pharmaceuticals (the Netherlands). It was developed for treating acute hepatic porphyria. Givlaari is comprised of synthetic double stranded siRNA oligonucleotide targeting the mRNA encoding delta-aminolevulinic acid synthase 1 enzyme. The siRNA is conjugated to a ligand containing 3 N-acetylgalactosamine residues targeting galactose receptor (Wang et al., 2018). Unlike Onpattro, to ensure stability, Givlaari has been modified to contain phosphorothioate backbone linkages at the termini and contains 2'-O-fluorourine and 2'-O-methyl groups in the pentose moiety. Givlaari has demonstrated the ability to significantly decrease the rate of porphyria attacks requiring hospitalizations, urgent healthcare or IV hemin administration.
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides. Site-specific labeling of RNA molecules is a valuable tool with multiple applications, and Bio-Synthesis, Inc. provides site-specific biotin labeling of long RNA at 5’-end.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/sirna-synthesis.aspx
https://www.biosyn.com/custom-rna-transcription-services.aspx
https://www.biosyn.com/tew/RNA-network-analysis-using-biotinylated-RNA-affinity-probes..aspx#
References
Agrawal N, Dasaradhi PV, et al. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev. 67: 657-85 (2003). PMID:14665679
Davis ME, Yen Y, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 464: 1067-70 (2010). PMID: 20305636
Li LC. Small RNA-Mediated Gene Activation. RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity. Caister Academic Press. ISBN 978-1-904455-25-7 (2008).
Tabernero J, Shapiro GI, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 3: 406-17 (2013). PMID: 23358650
Wang B, Rudnick S, et al. Acute Hepatic Porphyrias: Review and Recent Progress. Hepatol Commun 3:193-206 (2018) PMID: 30766957
Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 42:217-39 (2013). PMID: 23654304