For hybridization, the rate of renaturation is thought to be proportional to the square root of the length of the complementary single strands (Niranjani et al., 2016). In practice, shorter oligonucleotides are preferred as probes to achieve efficient delivery into cells or permeate across dense chromosomes to find target. To optimize, various parameters affecting hybridization such as probe length or concentration, temperature, and pH have been modulated. Another contributing factor is the electrostatic repulsion between the phosphate moieties of the two single strands of the duplex. Modifying this property affects hybridization as increasing the monovalent cation concentration (to neutralize the anionic phosphate groups) enhances the stability of the duplex. Likewise, lowering the salt concentration (to increase stringency) affects hybridization.
To attenuate the electrostatic repulsion, Zip Nucleic Acids (ZNA) was developed, consisting of oligonucleotides conjugated to positively charged spermine derivatives (Noir et al., 2008). It facilitates target recognition by allowing the oligonucleotide to scan the single stranded DNA till it finds the complementary sequence. Upon hybridization, the interaction of the positively charged moiety with complementary strand augments the binding affinity and stabilizes the duplex without compromising specificity, resulting in an increased Tm value. The ‘zipper’-like property enables ZNA primers to perform (more efficiently than LNAs) at lower concentrations of primer/Mg2+ and higher annealing temperatures, improving PCR amplification of AT-rich regions, RNA-to-cDNA conversion, and single-nucleotide polymorphism (SNP) discrimination (Moreau et al., 2009). The spermine modification renders oligonucleotide conjugates more resistant to nuclease degradation. ZNA-modified oligonucleotides were successfully used to detect miRNA expression via in situ hybridization (ISH) in maize seedlings (Trevisan et al., 2012).
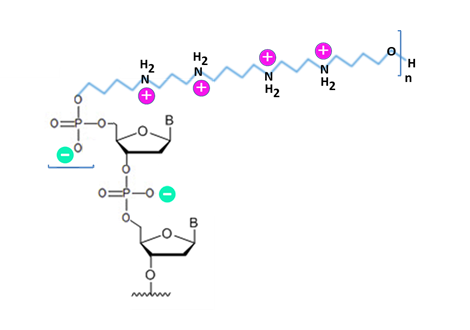
The potential of the positively charged moiety to mediate the transfer of ZNA oligonucleotides into cells was investigated. For antisense strategy involving RNA interference, oligospermine-oligoribonucleotide siRNA conjugates, which were resistant to nucleases in serum, entered HeLa cells without carrier to silence gene expression (Paris et al., 2012). Similarly, carrier-free ZNA-modified siRNA oligonucleotides (albeit non-tumor specific) entered A549Luc human lung carcinoma cells to suppress gene expression (Nothisen et al., 2009). For antigene strategy requiring triple helix formation to inhibit transcription, spermine conjugated LNA-modified oligonucleotides alone could enter fibroblasts (derived from Huntington's disease patients) to reduce huntingtin (HTT) expression by targeting its promoter (Gagnon et al., 2011).
Increasing the number of cationic units will alter the ‘overall charge’ of the ZNA oligonucleotides, with the resultant application of negatively charged conjugates for molecular biological/diagnostic applications and positively charged conjugates for enhanced target recognition (via potentially increasing ZNA’s attraction to nucleic acids) and the self-delivery of oligonucleotides into cells. To design ZNA oligonucleotides, its ‘net charge’ can be calculated using the equation “3n-(m-1)”, with m and n values representing the length of the oligonucleotide and the number of spermine units, respectively. To empirically determine the Tm value, the hybridization of dual complementary oligonucleotides with varying number of spermine units was examined (Noir et al., 2008). The results showed that, for 9 to 12-mer oligonucleotides, the modification raises Tm by 4 to 7 °C per spermine independent of the base sequence or conjugation site (5’ or 3′). The approximate Tm value of ZNA oligonucleotide can be calculated using the formula “Tm (ZNA™) = Tm (DNA) + 36z/(N-3.2)“, where z and N represent the number of cationic units and the number of nucleotides, respectively. Increasing the positive charge may reduce solubility, however, which could be resolved by raising pH or dissolving in concentrated PBS stock.
Bio-Synthesis, Inc. provides extensive options for the application of various modified nucleosides for research or therapy purposes. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including ZNA. For bridged nucleic acid (BNA), it has recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA. The BNA technology that we offer provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. More importantly, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides for clinical application.
References
Gagnon KT, Watts JK, Pendergraff HM, Montaillier C, Thai D, Potier P, Corey DR. Antisense and antigene inhibition of gene expression by cell-permeable oligonucleotide-oligospermine conjugates. (2011) J Am Chem Soc. 133(22):8404-7. doi: 10.1021/ja200312y. PMID: 21539318
Moreau V, Voirin E, Paris C, Kotera M, Nothisen M, Rémy JS, Behr JP,et al. Zip Nucleic Acids: new high affinity oligonucleotides as potent primers for PCR and reverse transcription. (2009) Nucleic Acids Res. 37(19):e130. doi: 10.1093/nar/gkp661. PMID: 19696078
Niranjani G, Murugan R. Theory on the Mechanism of DNA Renaturation: Stochastic Nucleation and Zipping. (2016) PLoS One 11(4):e0153172. doi: 10.1371/journal.pone.0153172. eCollection 2016. PMID: 27074030
Noir R, Kotera M, Pons B, Remy JS, Behr JP. Oligonucleotide-oligospermine conjugates (Zip Nucleic Acids): a convenient means of finely tuning hybridization temperatures. (2008) J Am Chem Soc 130:13500-5. doi: 10.1021/ja804727a. PMID: 18781743
Nothisen M, Kotera M, Voirin E, Remy JS, Behr JP. Cationic siRNAs provide carrier-free gene silencing in animal cells. (2009) J Am Chem Soc 131:17730-1. doi: 10.1021/ja908017e. PMID: 19928854
Paris C, Moreau V, Deglane G, Karim L, Couturier B, Bonnet ME, et al. Conjugating phosphospermines to siRNAs for improved stability in serum, intracellular delivery and RNAi-mediated gene silencing. (2012) Mol Pharm 9:3464-75. doi: 10.1021/mp300278b. PMID: 23148419
Trevisan S, Nonis A, Begheldo M, Manoli A, Palme K, Caporale G, et al. Expression and tissue-specific localization of nitrate-responsive miRNAs in roots of maize seedlings. (2012) Plant Cell Environ 35:1137-55. doi: 10.1111/j.1365-3040.2011.02478.x. PMID: 22211437