In trying to find an immediate intervention strategy to counter the COVID-19 pandemic, researchers have been focusing on developing antiviral vaccines. In the preceding article, various designs that have been to put forward for anti-COVID-19 vaccines have been described, which include recombinant virus (ex. adenovirus expressing COVID-19 protein) and inactivated virus (of COVID-19 itself). In the past, live attenuated viruses have been used successfully to control poliovirus and other viruses due to its ability to incite strong humoral (B-cell based) or cellular (T-cell based) immunity against the infectious agent. Nonetheless, its manufacturing can be time-consuming as it entails preparing a large scale of highly infectious virus, safeguarding against its contamination and/or its release, the need to stockpile, the potential for failure due to the acquiring of mutations by the virus, etc (Zhang et al., 2019). Recombinant viruses carry the risk of inciting immune reaction against the virus used as the delivery vector, which may provide a potential reason for the unexpected observation that recently developed adenovirus delivered COVID-19 vaccine against spike protein (Oxford University, England) was more effective at low dose than high dose (Callaway, 2020).
In this regard, nucleic acid-based vaccines could be ideal as they can be prepared in a short period of time without requiring an extensive knowledge regarding the biology of the virus. There are two types of the vaccines: DNA or mRNA--with the former being easier to prepare with less concern about the stability/degradation and lower costs. Upon internalization, the DNA vaccine must travel to the nucleus to be transcribed to mRNA (a process which may pose the risk of potential integration into the genomic DNA), which must then be exported to the cytoplasm to express antigen. Thus far, a limited success has been achieved with DNA vaccines in clinical trials presumably due to the insufficient 'strength' of the immunity induced (Kallen et al., 2014).
In contrast, mRNA vaccines only need to translocate across the cell membrane to allow the translation of the encoded antigen in the cytoplasm. The issue with low stability may be resolved through lyophilization as the lyophylized mRNA vaccines was reported to stay undegraded for a considerable duration at 25-40oC. As for the cost, mRNA vaccines could be made available at prices comparable to other vaccines based on protein, DNA, peptide, etc. The genetic target of mRNA vaccine could be switched from one to another easily and its production could be adapted to meet the GMP manufacturing standard.
The early works demonstrated that direct injection of DNA or RNA expression vectors into muscle could allow protein expression in mice (Wolff et al., 1990). This has inspired the development of vaccines comprised of mRNA-encoded antigens that could be delivered ex vivo or injected intradermally (underneath the skin). The 'ex vivo' approach refers to the process of excising tissues, transfecting with a vaccine construct (ex. via eletroporation) outside the body, and then returning it to the individual. The initial trial involved treating advanced-stage melanoma patients with dendritic cells electroporated with mRNA vaccines encoding MAAs (melanoma associated antigens), i.e. MAGE-A3, tyrosinase, MAGE-C2, gp100 (Wilgenhof et al. 2011).
Critical to this undertaking was the recognition of the role of dendritic cells in antigen presentation, the ability to expand dendritic cells in vitro, and the identification of tumor-associated antigens (Van Nuffel et al., 2010). The commonly used approach is to characterize the 'mutanome' (mutated gene sequences) via deep sequencing to identify mutated sequences that are highly immunogenic as mRNA vaccine. The antigen encoded could be chimeric as adding a signal peptide to N-terminus or an endosomal trafficking signal to C-terminus could improve antigenic presentation by MHC molecules (Kreiter et al., 2008). Further, the maturation status of dendritic cells could affect the uptake efficiency of mRNA vaccines {Kuhn et al., 2010).
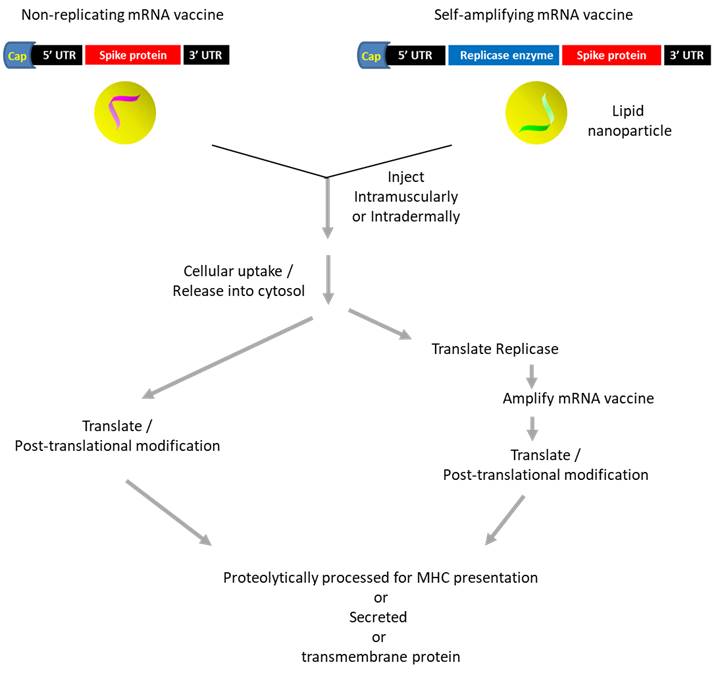
Several types of mRNA vaccines currently exist. The 'non-replicating mRNA' vaccine consists of the coding sequence (of the antigen) that is 5'-capped, 5' and 3' untranslated regions, and poly-A sequence. The use of T7 polymerase to generate mRNA transcript using linearized plasmid may generate double stranded RNAs due to self-priming (Triana-Alonso et al., 1996). The mRNA designed by Moderna Therapeutics (USA) incorporated modified nucleotides to avoid activating interferon-associated genes, and added 2 proline residues to stabilize the spike protein. While a lipid-based nanoparticle was used to protect mRNAs, delivery vector remains a significant issue. A phase I clinical trial (doses from 25 ug to 250 ug) showed that it induced antigen-binding and neutralizing antibodies (plus activating T cells) while causing side effects (ex. chills, fatigue, headache, muscle pain) which augmented after the second dose (Jackson et al., 2020). Phase III study (to determine if it can protect against COVID-19) is still ongoing. In contrast, the 'self-amplifying mRNA' vaccine is generated using alphavirus whose structural gene is replaced with an antigen of interest while retaining the genes mediating viral replication. Being a positive stranded RNA virus, it generates double stranded RNA as intermediates, which could potentially trigger innate immunity (ex. cytokine release) (Vogel et al., 2018). Alternatively, the construct may contain another cistron expressing replicase to amplify the mRNA vaccine (Jackson et al., 2020).
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. For medicinal chemistry, it specializes in peptide synthesis, characterization, modification, purification to generate various peptide-based building blocks as well as pharmaceutical intermediates—in addition to peptide libraries, peptide arrays, peptidomimetics. Antibody purification, characterization/quantification, modification and labeling are also offered. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/tew/mRNA-vaccines-and-innate-immunity.aspx#!
https://www.biosyn.com/tew/Messenger-RNA-(mRNA)-for-Vaccine-Development-Against-Coronavirus.aspx
https://www.biosyn.com/tew/Ribose-2’-O-methylation,-“self-and-non-self,”-and-Coronaviruses.aspx
References
Callaway E. Why Oxford's positive COVID vaccine results are puzzling scientists. Nature. Nov 23 (2020). PMID: 33230278
Jackson NAC, Kester KE, et al. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 5:11 (2020). PMID: 32047656
Kallen KJ, Theß A.Kallen KJ, et al. A development that may evolve into a revolution in medicine: mRNA as the basis for novel, nucleotide-based vaccines and drugs. Ther Adv Vaccines. 2:10-31 (2014). PMID: 24757523
Kreiter S, Castle JC, et al. Targeting the tumor mutanome for personalized vaccination therapy. Oncoimmunology. 1:768-769 (2012). PMID: 22934277
Kuhn AN, Diken M, et al. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 17:961-71 (2010). PMID: 20410931
Triana-Alonso FJ, Dabrowski M, et al. Self-coded 3'-extension of run-off transcripts produces aberrant products during in vitro transcription with T7 RNA polymerase. J Biol Chem 270:6298-307 (1995). PMID: 7534310
Van Nuffel AM, et al. Immunotherapy of cancer with dendritic cells loaded with tumor antigens and activated through mRNA electroporation. Methods Mol Biol 629:405-52 (2010). PMID: 20387165
Vogel AB, Lambert L, Kinnear et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol Ther. 26:446-455 (2018). PMID: 29275847
Wilgenhof S, Van Nuffel AMT, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 24: 2686-2693 (2013). PMID: 23904461
Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science 2471465-8 (1990). PMID: 1690918
Zhang C, Maruggi G, et al. Advances in mRNA Vaccines for Infectious Diseases. Front Immunol. 10:594 (2019). PMID: 30972078