Vaccination prevents many millions of illnesses and saves many lives every year. The smallpox virus vaccine is an excellent example of a successful vaccination approach.
Messenger RNA (mRNA) vaccines are a promising vaccine approach for high potency, rapid development, and potential low-cost manufacture and safe administration. mRNA is the intermediate between transcription and translation of a gene. A single strand of DNA is decoded by RNA polymerase during transcription, resulting in the biosynthesis of mRNA. mRNA transfers genetic information encoded in DNA to the ribosomal translational machinery in the cytoplasm to produce proteins.
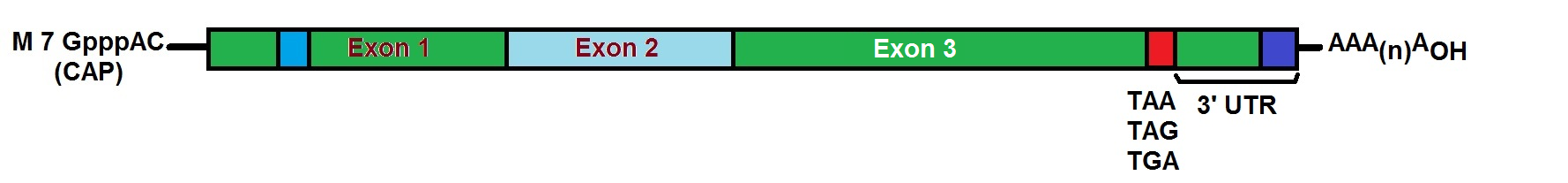
Figure 1: Mature messenger RNA.
Until recently, instability and inefficient in vivo delivery of mRNA restricted the application of mRNA vaccines. Recent technological advances help to overcome these issues. Multiple platforms against infectious diseases and several types of cancer no exist.
Classical vaccines that protect against many dangerous diseases include living attenuated and inactivated pathogens and subunit vaccines. However, for newly emerging viral infections, such as COVID-19, more rapid development and large-scale deployment is needed.
Nucleic acid-based therapies are promising alternatives to conventional approaches. Recently, mRNA became a promising therapeutic tool in vaccine development and protein replacement therapy.
Beneficial features for using mRNA as a vaccination platform are:
- Safety. mRNAs are non-infectious, non-integrating, with no potential risk of infection or insertional mutagenesis. Also, normal cellular processes degrade mRNA. Using a variety of modifications and delivery methods allow regulating the in vivo half-life of mRNA. For increased safety, inherited immunogenicity of mRNA needs to be downregulated.
- Efficacy. A variety of modifications allow increasing mRNA stability and translatability to produce a desired and satisfactory mRNA product. To ensure improved in vivo delivery, formulating mRNA into carrier molecules allows rapid uptake and expression in the cytoplasm. The use of mRNA-based vaccines avoids anti-vector immunity and enables repeated administration.
- Productions. Messenger RNA vaccines potentially allow rapid, inexpensive, and scalable manufacturing due to the high yields of in vitro transcription reactions.
Recent results suggest that mRNA vaccines potentially solve many challenges in vaccine development for infectious diseases and cancer.
Production of optimally translated in vitro transcript mRNA
Starting from a linear DNA template with the help of T7, a T3 or an Sp6 phage RNA polymerase, in vitro transcription (IVT) produces mRNA. The IVT product needs to contain an open reading frame (ORF) that encodes the target protein, flanking untranslated regions (UTRs), a 5’-cap and a poly(A) tail. Engineering of the mRNA results in a fully processed mature mRNA molecule as it occurs naturally in the cytoplasm of eukaryotic cells.
Extracellular RNases quickly degrade naked mRNA. Naked mRNA is also not efficiently internalized. To solve this problem, newly developed in vitro and in vivo transfection reagents facilitate cellular uptake of mRNA and protect it from degradation. After successful uptake and transit of mRNA into the cytosol, the cellular machinery produces protein. Post-translational modifications result in a correctly folded, functional protein. Finally, the protein is delivered to the correct cellular compartment by normal physiological processes for proper presentation or function. Degradation by normal physiological processes reduces the risk of metabolite toxicity.
Exogenous or cell foreign mRNA can stimulate an immune response. A variety of cell surfaces recognize foreign RNA through endosomal and cytosolic innate immune receptors. This effect of RNA on the cell can be beneficial or detrimental. Vaccination can drive dendritic cells (DCs) to maturity to elucidate a robust T and B cell responses. Unfortunately, innate immune sensing of mRNA can also inhibit antigen expression, thereby negatively affecting the desired immune response.
mRNA and Innate Immunity
How innate immune sensing works is not well understood. IVT mRNA show an immunostimulatory profile that can be shaped by purification, the introduction of modified oligonucleotides, and the complexing of mRNA with a variety of carrier molecules. Enzymatically synthesized mRNA contains double-stranded (dsRNA) contaminations. Pattern recognition receptors present in multiple cellular compartments sense dsRNA as a potent pathogen-associated molecular pattern (PAMP). IVT mRNA that contains dsRNA results in robust type I interferon production. Type I interferon upregulates the expression and activation of protein kinase R (PKR or EIF2AK2) and 2’-5’-oligoadenylate synthetase (OAS), resulting in inhibited translation, and degradation of cellular mRNA and ribosomal RNA. However, chromatographic purification methods allow efficient removal of contaminated dsRNA. Both reversed-phase fast protein liquid chromatography (FPLC) or high-performance liquid chromatography (HPLC) remove dsRNA to achieve a highly pure product. The result is an increased protein production in primary human dendritic cells from IVT mRNAs.
Externally delivered single-stranded RNAs are also PAMPs. Endosomal Toll-like receptors 7 (TLR7) and TLR8 sense degradation products of RNAs resulting in type I interferon production. Incorporation of naturally occurring chemically modified nucleosides, including pseudouridine and 1-methylpseudouridine as well as others, prevents RNA sensing by TLR7, TLR8, and other innate immune sensors, resulting in a reduction of type I interferon signaling. Nucleoside modification also partially suppress the recognition of dsRNA species.
Recent studies showed increased in vitro translation of nucleoside-modified mRNA compared to unmodified mRNA, which is also the case in vivo in mice. High levels of protein production can be achieved in DCs using purified and nucleoside-modified mRNA.
These findings advanced our understanding of innate immune sensing and how to avoid the adverse effects of innate immunity.
Lipid-encapsulated or naked forms of sequence-optimized mRNA vaccines can potentially produce potent vaccines against a variety of viruses such as SARS-CoV-2, influenza virus, Zika virus, rabies virus, and many others.
Different types of mRNA vaccines are cancer vaccines, dendritic cell vaccines, and various kinds of directly injectable mRNA vaccines.
However, for therapeutic considerations, good manufacturing practice (GMP) production must be established to guarantee the safety and increased efficacy of mRNA vaccines.
Reference
Pardi, N., Hogan, M., Porter, F. et al., 2018; mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018). [nature]
World Health Organization Immunization for Diseases
.png)
---...---