Photoisomerization is the photochemical cis-trans isomerization of organic molecules with a double bond in their structure. This reaction is a common photo-reaction. During photoisomerization one isomer is converted into another by light. Photoisomerization is a behavior of molecules in which a structural change between isomers is caused by photoexcitation. For some molecules, both reversible and irreversible isomerization reactions exist, however, photoisomerization usually indicates a reversible process.
Dichloroethylene is an example of a molecule for which a laser-induced isomerization is known. Nagai and Katayama observed the infrared induced photoisomerization of both trans- and cis-1,2-dichloroethylene molecules sensitized by SF6 using a CO2 laser.

Light with the proper energy can convert one isomer into another. A very nice explanation for this reaction can be found here.
https://chemistry.stackexchange.com/questions/31730/photoisomerization-of-azobenzene.
Other examples are cis-stilbene which can be converted to trans-stilbene, and O-methyl oximes.
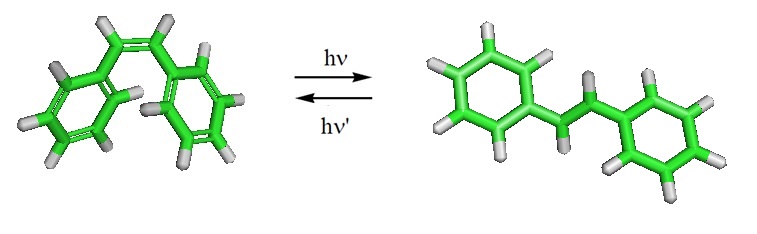
Stilbene can undergo photoisomerization under the influence of UV light. Stilbene can also undergo photocyclization. Many stilbene derivatives called stilbenoids are present in plants. Resveratrol and pterostilbene are examples.
For stilbene, the potential energy surface of the S1 state has been optimized and scanned using a multistate multiconfiguration second-order perturbation theory. Also, the trans-stilbene, pyramidalized (phantom), and DHP-cis-stilbene geometric domains of the S1 state were characterized, and their stationary points including the transition states between them, as well as S1/S0 intersections were described. Furthermore, the minima, the activation barriers in the ground state, the dynamic behavior of excited cis-stilbene, and the trans-cis branching ratio after relaxation to S0 through a unsymmetric conical intersection were reported.
Azobenzene, a molecule used in biotehcnological applications, can undergo a characteristic trans-cis or cis-trans photoisomerization reaction.
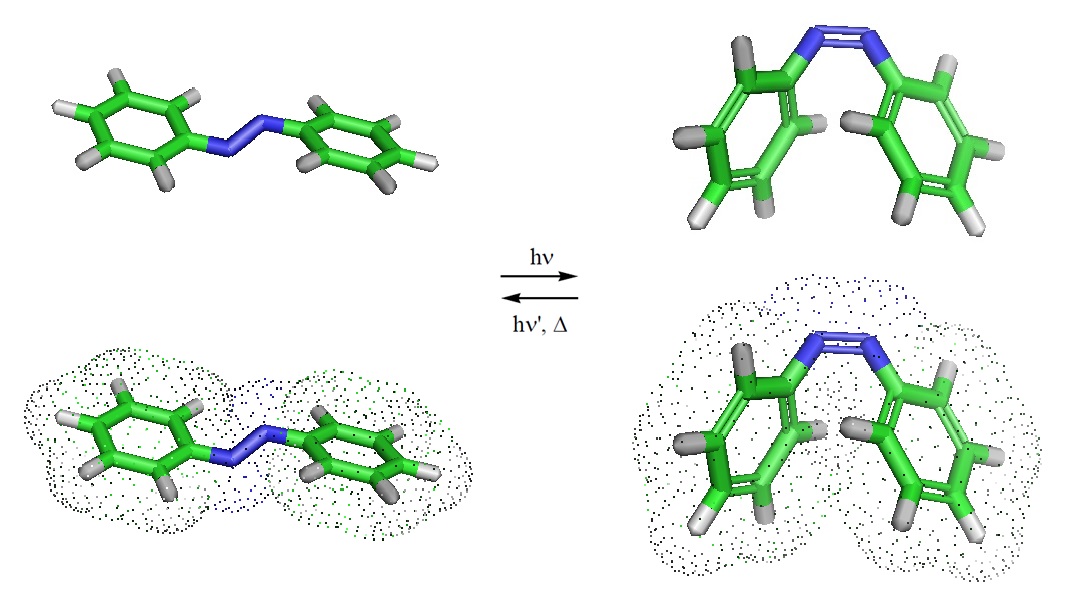
Isomerization takes place because the excited states, both S1 and T1, of many alkenes have a perpendicular instead of a planar geometry. Cis-trans isomerization disappears upon excitation, but either isomer can be formed when the excited molecule drops back to the S0 state. Alkenes can be excited via the π→ π* pathway which is an important excitation pathway in organic chemistry. Molecules in the S1 state can drop to some low vibrational level of the S0 state by giving off light. This process usually happens within 10-9 seconds and is called fluorescence. Simulation studies suggest that the mechanical isomerization first proceeds through a mechanically activated inversion after which it is diverted to an ultrafast downhill rotation that accomplishes the isomerization process.
Reference
I. N. Ioffe and A. A. Granovsky; Photoisomerization of Stilbene: The Detailed XMCQDPT2 Treatment. Journal of Chemical Theory and Computation 2013 9 (11), 4973-4990. DOI: 10.1021/ct400647w. http://pubs.acs.org/doi/abs/10.1021/ct400647w.
March’s Advanced Organic Chemistry. 6th edition page 346.
Keeichi Nagai, Mikio Katayama; CO2 laser induced isomerization and photodissociation of 1,2-dichloroethylene. Chemical Physics Letters 51, 2, 329-332. https://doi.org/10.1016/0009-2614(77)80414-4. http://www.sciencedirect.com/science/article/pii/0009261477804144.
Junfeng Shao, Yibo Lei, Zhenyi Wen, Yusheng Dou, and Zhisong Wang; Nonadiabatic simulation study of photoisomerization of azobenzene: Detailed mechanism and load-resisting capacity. The Journal of Chemical Physics 129, 164111 (2008); doi: http://dx.doi.org/10.1063/1.3000008.
---...---