Carbamylation or homocitrullination is a post-translational modification (PTM) known for many years in the context of uremia, a metabolic disorder caused by the accumulation of waste products in the blood normally excreted in urine.
Carbamylation involves the non-enzymatic reaction of urea-derived cyanate with free NH2- groups on lysine (K, Lys) residues in proteins to yield homocitrulline (Hcit). Protein N-termini can also be carbamylated. Carbamylation can also be artificially introduced during sample preparation using urea.
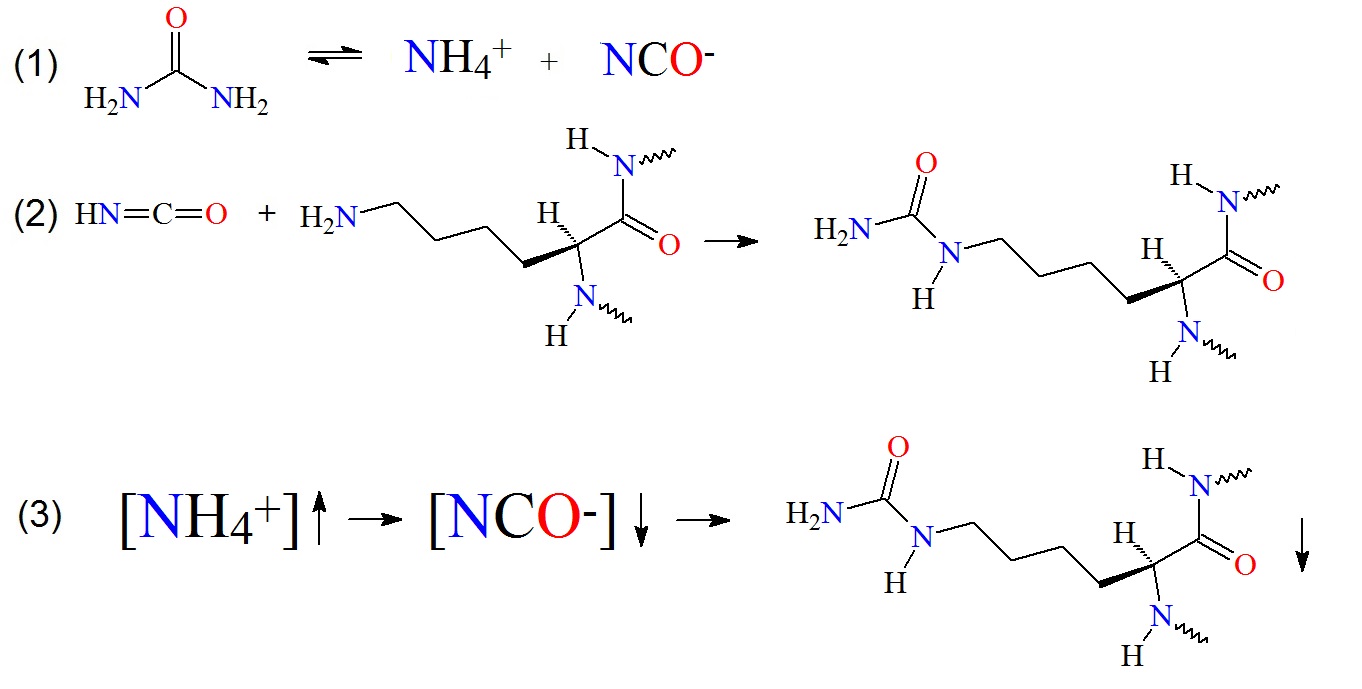
Figure 1: Carbamylation of amino groups on lysine residues. (1) Urea decomposition occurs in aqueous solutions. (2) The released cyanate can now react with free amino groups in proteins, peptides or lysine to carbamylate the molecules. (3) The relationship between ammonium ion, cyanate concentrations and protein carbamylation in fixed urea solution concentration and temperature is illustrated as proposed by Sun et al. in 2014.
Carbamylation adds an NHCO group to lysine to convert it to homocitrulline. Carbamylated proteins and peptides can be detected by a mass increase of the modified peptide by a delta mass of 43 mass units when detected in a mass spectrometer. If the ratio of homocitrulline is high in proteins or peptides, amino acid analysis may be used for its analysis. However, for the detection of low amounts of homocitrulline in tissue or protein mixtures, selective enrichment of the modified peptides followed by analysis using nano-spray liquid chromatography tandem mass spectrometry (LC-MS/MS) will be the analysis of choice.
Isocyanic acid (CNOH, ΔM 43) is derived from spontaneously dissociating urea forming cyanate and ammonia in aqueous solutions. Temperature, incubation time and pH are factors that affect the rate of urea dissociation and the degree of protein/peptide carbamylation.
Urea is commonly used for denaturing proteins in aqueous solutions. In these solutions, urea is in equilibrium with ammonium and isocyanate. Under alkaline conditions isocyanate can react with primary amines of free N-terminal group and ε-amino groups of lysines in proteins and peptides to form carbamylates. Prolonged incubation of proteins in urea buffers can introduce unwanted carbamylation by-products and thereby interfere with modern proteomics workflows including stable isotope-labeling methods.
A detailed analysis of urea stability in different solutions by Panyachariwat and Steckel showed that urea is more stable at a pH range of pH 4 to 8. However, the stability of urea decreased with increased temperature for all pH values. The lowest urea degradation occurred in lactate buffer at pH 6.0. Furthermore, urea decomposition rates in solution and pharmaceutical preparations depend on the initial urea concentration. At a higher initial concentration of urea, the degradation rate is lower.
Table 1: Residue and Delta Masses
|
Amino Acid
|
Residue Composition
|
Residue
Monoisotopic Mass
|
Delta Mass
|
|
Lysine
|
C6H12N2O
|
128.09496
|
0
|
|
Carbamyl Lysine
|
C7H13N3O2
|
171.10078
|
43.00582
|
|
Carbamylation
|
* NHCO
|
43.00582
|
-
|
|
*Note: A proton is lost from the amino group on the protein during carbamylation. The change in composition is NHCO. {Source: Ionsource}
|
Gorisse et al. in 2016 could show that carbamylation is associated with aging and life expectancy in mammalian species including humans. Carbamylation promotes molecular aging through alteration of protein functions such as of long-lived extracellular matrix proteins. Accumulation of carbamylated proteins in tissue is now considered as a general hallmark of aging.
Carbamylation in the skin
Carbamylation-derived products (CDPs) have been found to accumulate in the skin with age. Carbamylation occurs throughout the whole lifespan of mammals as well as in humans and leads to the accumulation of carbamylated proteins in tissues. Matrix proteins such as type I collagen and elastin are preferentially carbamylated. Homocitrulline accumulates more intensely than carboxymethyl-lysine. However, carboxymethyl-lysine is one of the major glycation end products. Hence, carbamylation may play a more prominent role in age-related tissue alterations then do glycoxidation reactions. Furthermore, it is known that carbamylated proteins are present in sera of patients with rheumatoid arthritis (RA) and can be used to predict joint damage. Shi et al. in 2011 reported that autoantibodies are present in sera of RA patients that recognized carbamylated proteins. The presence of IgG antibodies that recognize carbamylated antigens were found in 45% of RA patients.
Protein carbamylation rates
Protein carbamylation rates can be detected and analyzed in skin extracts by quantification of homocitrulline (HCit) or homocitrulline residues. The study by Gorisse et al. revealed that during aging, HCit concentrations significantly increase with age in all species studied. For example, HCit increased from 0.08 mmol/mol Lys in 1-d-old mice up to 2.2 mmol/mol Lys in 2-y-old mice, representing a 29-fold increase. Type I collagen carbamylation increased in all species as well, and human elastin also showed an age-dependent increase in HCit content. HCit content did not exceed 2.5 mmol/mol Glu in younger subjects (<4 y old), but it reached 11.7 mmol/mol Lys in older subjects (>70 y old), representing a 4.7-fold increase.
Urea in skin creams
Urea is also used in many skin creams to keep the creams and skin moist. However, stability studies of urea indicate that skin cream may need to be rather formulated in buffers containing ammonia or tertiary amines at pH 4 to 6 or above pH 7, if possible, to prevent carbamylation of skin proteins. Adding certain amino acid may also be beneficial.
Carbamylation and myeloperoxidase
In tissue, carbamylation can also be mediated in vivo by myeloperoxidase (MPO), the enzyme responsible for the inflammation-driven carbamylation of proteins via the MPO/H2O2/SCN- system. MPO has recently attracted attention as a potential trigger factor for atherogenesis and inflammation.
Apparently MPO has a negative impact on the extent of adaptive immune responses, but does not inhibit proinflammatory processes.
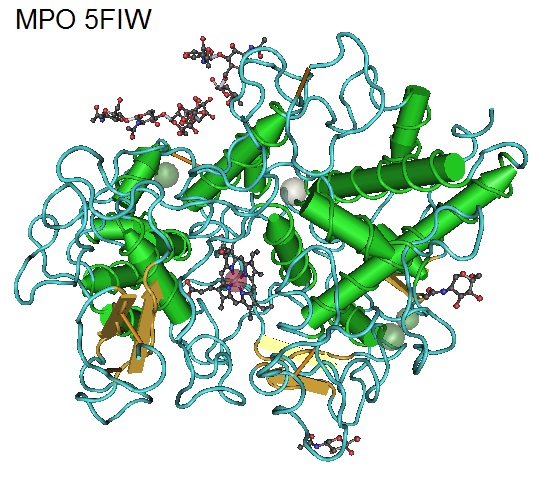
Figure 2: Structure of Human Myeloperoxidase 5FIW. Myeloperoxidase (MPO) is known as a front-line defender against phagocytosed microorganisms. MPO utilizes H2O2 to generate hypochlorous acid (HClO) and other reactive species to kill pathogens during infections but can also promote inflammation and causing tissue damage. Elevated levels of MPO have been observed in autoimmune diseases such as in the central nervous system (CNS) of multiple sclerosis (MS) and in the joints of rheumatoid arthritis (RA) patients.
Additionally, carbamylation of serum albumin has been implicated as a risk factor for mortality in patients with kidney failure (Berg et al. 2013).
In biochemistry and proteomics, urea solutions are the most widely used as denaturants. When proteins are digested in the presence of urea carbamylation can occur.
What is the result of carbamylation?
(1) Carbamylation block N-terminal ends of proteins and peptides and react with
the amino acid side chains of lysine and arginine residues. Hence, carbamylated
amino groups are now no longer available for downstream labeling reactions.
(2) Carbamylated amino groups prevent enzymatic digestions resulting in
incompletely digested peptides.
(3) Carbamylated proteins and peptides have different and sometimes unexpected
retention times when studied using chromatography or SDS-PAGE as well as
increased and unpredicted masses. This increases the complexity of samples.
(4) Carbamylation affects peptide and protein identification as well as quantification.
(5) In vitro-carbamylation affects the study of in-vivo carbamylation as being
observed in metabolic diseases such as in uremia, and severe renal and
cardiovascular disorders.
For the study of in-vivo carbamylated molecules, carbamylation reactions occurring during sample handling steps need to be prevented.
How can carbamylation of proteins and peptides be prevented?
Sun et al. recently showed that NH4HCO3 buffer is more effective in protecting proteins and peptides against carbamylation in urea solution than phosphate (PB) or Tris-HCl buffer. The research group found that the inhibition efficiency increased with increased NH4HCO3 concentrations and that a 1M NH4HCO3 buffer nearly completely prevented carbamylation on proteins and peptides during tryptic digestion in urea solution without inhibiting trypsin. Also, other ammonium or tertiary ammonium containing buffers also inhibited protein carbamylation during protein digestion in urea solution.
Reference
Berg, A. H., Drechsler, C., Wenger, J., Buccafusca, R., Hod, T., Kalim, S., … Karumanchi, S. A. (2013). Carbamylation of Serum Albumin as a Risk Factor for Mortality in Patients with Kidney Failure. Science Translational Medicine, 5(175), 175ra29. http://doi.org/10.1126/scitranslmed.3005218
Gorisse, L., Pietrement, C., Vuiblet, V., Schmelzer, C. E. H., Köhler, M., Duca, L., Gillery, P. (2016). Protein carbamylation is a hallmark of aging.Proceedings of the National Academy of Sciences of the United States of America, 113(5), 1191–1196. http://doi.org/10.1073/pnas.1517096113.
https://pubchem.ncbi.nlm.nih.gov/compound/Isocyanic_acid#section=Top,
http://pubs.acs.org/doi/abs/10.1021/ja01623a011
Kollipara L, Zahedi RP.; Protein carbamylation: in vivo modification or in vitro artefact? Proteomics. 2013 Mar;13(6):941-4. doi: 10.1002/pmic.201200452.
Panyachariwat N, Steckel H.; Stability of urea in solution and pharmaceutical preparations. J Cosmet Sci. 2014 May-Jun;65(3):187-95.
William H. R. Shaw, John J. Bordeaux; The Decomposition of Urea in Aqueous Media. J. Am. Chem. Soc., 1955, 77 (18), pp 4729–4733. DOI: 10.1021/ja01623a011. https://www.ncbi.nlm.nih.gov/pubmed/25043489
Jing Shi, Rachel Knevel, Parawee Suwannalai, Michael P. van der Linden, George M. C. Janssen, Peter A. van Veelen, Nivine E. W. Levarht, Annette H. M. van der Helm-van Mil, Anthony Cerami, Tom W. J. Huizinga, Rene E. M. Toes, and Leendert A. Trouw; Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage PNAS 2011 108 (42) 17372-17377; published ahead of print October 10, 2011, doi:10.1073/pnas.1114465108.
Strzepa, A., Pritchard, K. A., & Dittel, B. N. (2017). Myeloperoxidase: A new player in autoimmunity. Cellular Immunology, 317, 1–8. http://doi.org/10.1016/j.cellimm.2017.05.002 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5665680/
Sun, S., Zhou, J.-Y., Yang, W., & Zhang, H. (2014). Inhibition of Protein Carbamylation in Urea Solution Using Ammonium Containing Buffers.Analytical Biochemistry, 446, 76–81. http://doi.org/10.1016/j.ab.2013.10.024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4072244/
---...---