The mechanism by which molecules move across the cell’s nuclear membrane is called “nuclear transport.” Nuclear pore complexes control the entry and exit of larger molecules.
To move across the nuclear membrane, biomolecules such as plasmid DNA, RNA and proteins need association with transport factors called nuclear transport receptors. Nuclear pore complexes (NPC) form a selectively permeable barrier between the cytoplasm and the nucleus permeable to molecules smaller than ~40 kDa. However, most large molecules cross through the NPC in an energy-dependent process. Soluble transport factors of the importin β-superfamily (also known as β-karyopherins) mediate the transport.
Enhancing drug delivery of therapeutic drugs will increase their efficacy. After delivery, Most drugs end up in endo/lysosomal vesicles, not the nucleus. From there, drugs need to escape from vesicles into the cytoplasm and translocate into the nuclei. Cells have many intracellular resistance mechanisms cytosolic drugs must overcome. As a result, only a small portion of the drug delivered into the cytosol finally reaches the nucleus, particularly in drug-resistant cells. Many research groups now work to enhance the specific delivery of therapeutic drugs to their cellular target.
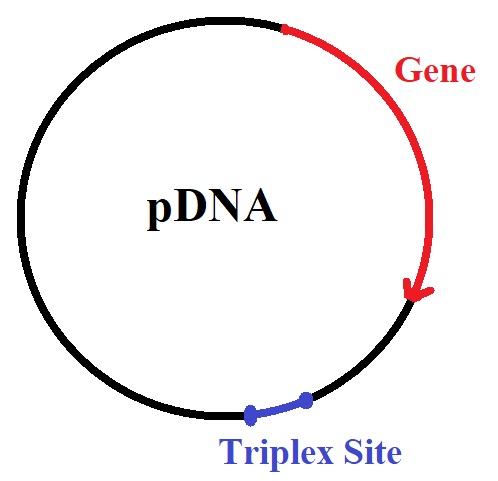
Signal molecules attached to plasmid DNA (pDNA) enable guided delivery of pDNA to the nucleus. A peptide-bridged nucleic acid (BNA)-pDNA construct is a vehicle for nonviral gene therapy.
Triplex forming bridged nucleic acid (BNA) oligonucleotides conjugated to peptides enable the delivery of plasmid DNA to the nucleus with increased transfection efficiency. This BNA-oligonucleotide-peptide conjugate allows selective intracellular targeting and expression of pDNA-encoded genes.
The conjugation of triplex-forming bridged nucleic acid oligonucleotides to a microtubule interacting peptide enabled the attachment of the peptide conjugate to plasmid DNA (pDNA). The microtubule interacting peptide (MTP) guides the delivery of pDNA to the nucleus along microtubule tracks.
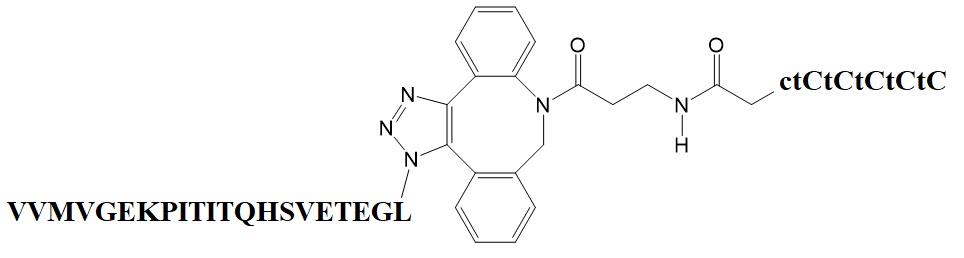
Girardin et al. recently reported a method for the design and synthesis of a BNA-based triplex-forming oligonucleotide conjugate. Using click chemistry, the researchers conjugated the BNA-triplex forming oligonucleotide to a microtubule protein targeting peptide. The peptide-linked BNA oligonucleotide also targets triplex-forming oligonucleotides with oligopurine • oligopyrimidine sites in pDNA. The triplex-forming sequences are inserted into the pDNA outside the luciferase gene sequence.
For the synthesis of the triplex-forming oligonucleotide, the oligonucleotide CTCTCTCTCTC was modified with BNAs to yield the BNA oligonucleotide C+TC+TC+TC+TC+TC in which N+ indicates the position of the BNA placement.
|
|
-NNNNN-(TCTCTCTCTCTC)n-NNNNNNNNN---
ctCtCtCtCtCt-linker-Peptide
NNNNN-(AGAGAGAGAGAG)N-NNNNNNNNN---
|
|
pDNA: Girardin et al. used rpDNA of 5 kbp encoding the luciferase gene and a pDNA of 21 kbp encoding both the luciferase and the full-length dystrophin genes. In vivo transfection was performed by Hydrodynamic Limb Vein (HLV) injection of the large pDNA encoding both the luciferase and the dystrophin genes.
|
Triplex forming sequences linking the peptide to the pDNA.
|
|
|
|
BNA-oligonucleotide-peptide conjugate formed by click chemistry.
Linkage of the peptide to pDNA: the linkage occurs via a BNA oligonucleotide-peptide conjugate forming a triple helix with pDNA containing triplex forming sites.
The peptide containing amino acid residues 79 to 98 from the amino acid sequence of the adenovirus E3 early protein of 14.7 kDa (E3-14.7K) specifically interacts with FIP-1 binding to the dynein light chain TCTEL-1. The peptide mediates interaction of pDNA with microtubules. The interaction of the protein complex promotes migration of the pDNA along microtubules toward the nucleus.
N = BNA, n = natural nucleic acid.
|
As a side note: There are two known microtuble motor proteins, kinesins and dyneins. Kinesins (with the exception of kinesin 14) move towards the (+) end of MTs to the cellular periphery. Dynein moves towards the (−) end in the direction of the cell nucleus.
Reference
Girardin, C., Maze, D., Gonçalves, C. et al. Selective attachment of a microtubule interacting peptide to plasmid DNA via a triplex forming oligonucleotide for transfection improvement. Gene Ther (2022). [nature]
Strambio-De-Castillia, C., Niepel, M. & Rout, M. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol 11, 490–501 (2010). [nature]
BNAs
BNAs-as-Tools-for-DNA-or-RNA-Targeting
BNAs as Molecular Tools
BNAs-for-duplex-and-triplex-formation
A-2-4-bridged-nucleic-acid-containing-2-pyridone-as-a-nucleobase-efficient-recognition-of-a-c-g-interruption-by-triplex-formation-with-a-pyrimidine-motif
Triplex-Formation-for-the-Detection-of-microRNAs
Triplex-Medicated-Gene-Modification
Highly-stable-pyrimidine-motif-triplex-formation-at-physiological-ph-values-by-a-bridged-nucleic-acid-analogue
Promotion-of-triplex-formation-by-bna-nc-modification
Molecular Motors in Molecular Biology of the Cell. 4th edition.
---...---
Bio-Synthesis provides a full spectrum of oligonucleotide and peptide synthesis including bio-conjugation services as well as high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---