Despite the advances that have been made in molecular medicine, many of the centuries-old diseases continue to persist. These include genetic disorders such as cancer, diabetes, and immunological diseases (ex. autoimmune disorders such as arthritis) as well as infectious diseases like malaria suffered by King Tut (Egyptian pharaoh Tutankhamen during 1332–1323 BC). Despite the temporary containment of microbial diseases, the continued emergence of drug-resistant strains and novel pathogenic strains via molecular evolution at the genomic level [ex. HIV (human T-lymphotropic virus-III), Covid-19 (SARS-CoV-2) coronavirus] undermines the efficacy of therapeutics time after time.
To meet these challenges, there has been a steadfast demand for pharmaceutical industries to develop innovative therapeutics. However, the exorbitant cost associated with both the development and clinical testing of novel drugs presents an unprecedented dilemma. To resolve this, some industries have resorted to repurposing previously FDA-approved drugs—that is, if they could meet mechanistic requirements.
Nevertheless, many of these drugs (ex. anticancer chemotherapeutics) display acute or delayed side effects. Hence, for their re-utilization, a molecular means to deliver them selectively to diseased cells (whether from normal organs or cancerous tissue) is increasingly sought. To fulfill this role, various biological entities have been examined, which include antibodies, peptides, lipids as well as sugar molecules, ex. N-acetylgalactosamine (GalNAc) (Ranasinghe et al., 2022). Even the possibility of using synthetic organic polymers (ex. polyester) to fabricate nanoparticles has been suggested (Yan et al. 2016). As the composition of the guiding agent may differ significantly from that of the therapeutic payload, the technique of conjugating chemically without disrupting their functions has become an area of great priority.
For conjugating to biological molecules, several commonly occurring reactive groups have been exploited. For instance, the -NH2 group, which occurs N-terminally as well as in the side chains (ex. lysine) could be used to link to various biomolecules bearing functional groups. The sulfhydryl group of cysteine represents another reactive moiety. Carboxylic groups (ex. glutamic acid) could react with various functional groups to enable conjugation. Nonetheless, the frequent occurrence of these residues in proteins has made it difficult to control the number of drugs being conjugated per biomolecule (ex. antibody), resulting in a heterogeneous mixture of conjugates with diverse therapeutic and pharmacokinetic properties.
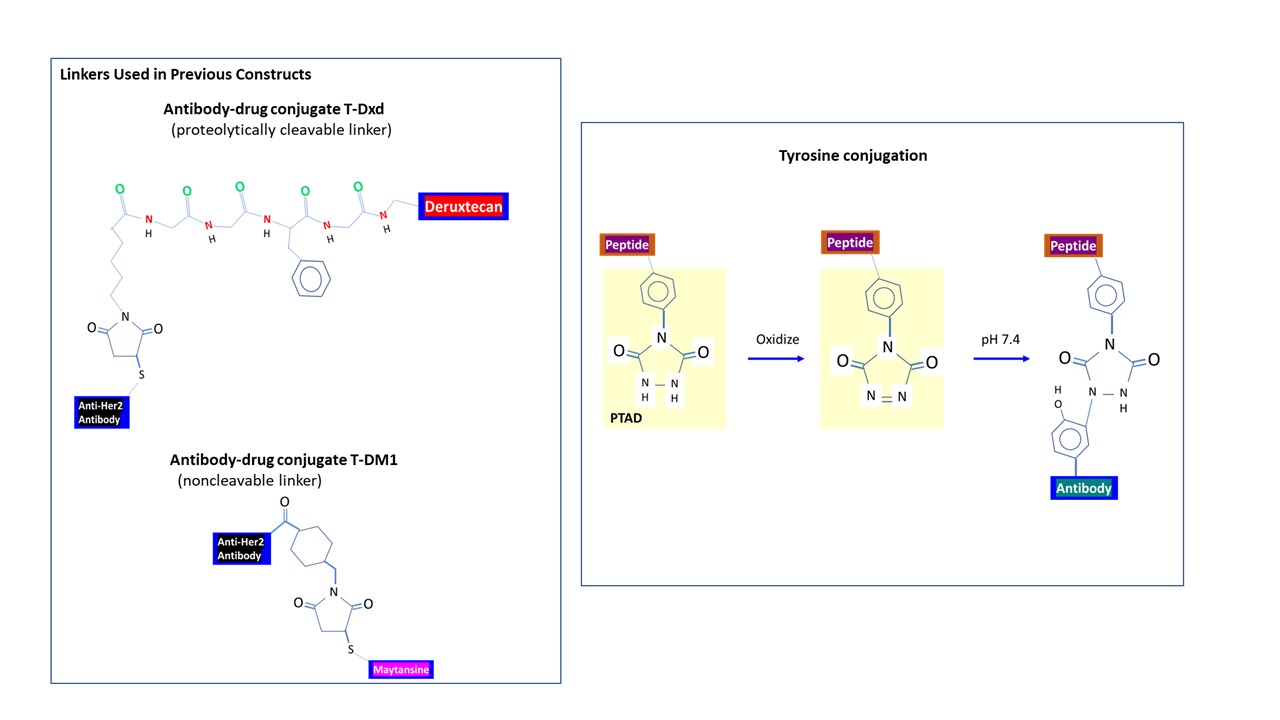
Further, conjugating to sulfhydryl groups may require reducing the interchain disulfide bridge a priori, which may alter the overall 3-dimensional structure of a protein. Conjugates incorporating thiosuccinimide may not be as stable (Szijj et al., 2018) though it’s present in several FDA-approved antibody-drug conjugates (ADCs), ex. Trastuzumab emtansine (T-DM1; Genentech of Roche Pharmaceuticals, Switzerland), Trastuzumab deruxtecan (T-Dxd; AstraZeneca Pharmaceuticals, United Kingdom). Whereas the linker used to generate T-DM1 is uncleavable, the linker for T-Dxd is proteolytically cleavable. The latter ADC T-Dxd consisting of the antibody Herceptin (recognizes Her2 receptor) conjugated to topoisomerase I inhibitor has shown efficacy for treating metastatic breast cancer despite side effects. Her2 is also expressed in normal cardiac tissues.
Other amino acids being considered for bioconjugation include arginine, tryptophan, methionine, and tyrosine. The side group of tyrosine consists of a phenyl ring and a hydroxyl group (may form H-bond). Unlike lysine, tyrosine (which occurs at a moderate frequency) is often buried from the surface of the protein and could be used for site-specific conjugation (Dorta et al., 2020). The concern over protein dimerization caused by the highly reactive -SH group of cysteine may not apply to tyrosine, which is nevertheless sufficiently reactive for bioconjugation. A widely used conjugation method employs PTAD [4-phenyl-3H-1,2,4-triazoline-3,5(4H)-dione], which reacts selectivity with tyrosine even in the presence of histidine, tryptophan, or lysine. PTAD must be oxidized from its precursor immediately before reaction as it is unstable under physiological condition (Szijj et al., 2020). One caveat is its tendency to decompose upon exposure to water and form isocyanate product (reacts with an amino group to generate side product), which may entail the use of an isocyanate scavenger.
Alternatively, tyrosine could be selectively modified via a ‘Mannisch-type reaction’ (imine formed from aniline and aldehyde may react with tyrosine). Further, tyrosyl radicals generated via catalysis by transition metal complexes could be used to achieve tyrosine O-alkylation. Sulfur fluoride exchange represents another method for conjugating to tyrosine, wherein fluoride (of sulfur fluoride) is displaced by an O-nucleophile (of tyrosine) to form a conjugate. Oxidation of tyrosine may be achieved enzymatically if buried tyrosine could be accessed. To externally engraft tyrosine at the surface of an antibody, a method involving recombinant protein expression plus tubulin tyrosine ligase was described (Creative Biolabs, Inc.).
The key to preventing an epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogs (over ~200) including bridged nucleic acid (BNA) in addition to mRNA synthesis. A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as to conjugate to peptides or antibodies. It provides custom conjugation of small molecules such as chemical drugs, metabolites and labeled compounds with synthetic or natural polymers (enzymes, peptide, protein, oligonucleotide, antibody, dendrimer, nanoparticle, etc). It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, the third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. It has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple-negative breast cancer. The BNA technology provides superior, unequaled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides. For therapeutic consideration, peptide modifications may include labeling, conjugation, cyclization, incorporation of unusual amino acids, and modification of side chain and backbone.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/mrna.aspx
https://www.biosyn.com/tew/Design-Guidelines-for-BNA-based-Oligonucleotide-Probes.aspx#!
https://www.biosyn.com/bioconjugation.aspx
https://www.biosyn.com/tew/Basic-Bioconjugation-Chemistry-of-Reactive-Groups-in-Biomolecules.aspx
https://www.biosyn.com/tew/Bioconjugate-Chemistry-for-Molecular-Engineering.aspx#!
References
Dorta DA, Gouin SG, et al. Tyrosine Conjugation Methods for Protein Labelling. Chemistry. 26:14257-14269 (2020). PMID: 32538529
Ranasinghe P, Webb DJ, et al. Small interfering RNA (siRNA): discovery, pharmacology and clinical development - an introductory review. Br J Pharmacol. Oct 17, 2022. PMID: 36250252
Szijj PA, Chudasama V, et al. Minireview: Addressing the retro-Michael instability of maleimide bioconjugates. Drug Discov Today Technol. 30: 27-34 (2018). PMID: 30553517
Yan Y, Siegwart DJ, et al. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc Natl Acad Sci USA. 113: E5702-10 (2016). PMID: 27621434