What is protein tyrosine O-sulfation?
Protein tyrosine-O-sulfation is a post-translational modification that occurs in most eukaryotes. Tyrosine-O-sulfation was discovered by Bettelheim in 1954 in the bovine protein fibrinogen. Baeuerle and Huttner in 1987 showed that tyrosine sulfation is a frequent modification of secretory proteins that occurs in the trans-Golgi apparatus. The heavy chain of immunoglobulin M (IgM) produced by hybridoma cells was found to contain tyrosine sulfate. Their data suggested that within the trans-Golgi, tyrosine sulfation of IgM occurred at least in part after terminal glycosylation. This modification, therefore, appeared to be the last modification of this constitutively secreted protein before its exit from this compartment. These results establish the covalent modification of amino acid side chains as a function of the trans-Golgi.
Tyrosine sulfation is mediated by one of only two tyrosyl-protein sulfotransferases (EC 2.8.2.20), TPST1 and TPST2 in mice and humans. Figure 1 illustrates the tyrosylprotein sulfotransferase reaction.
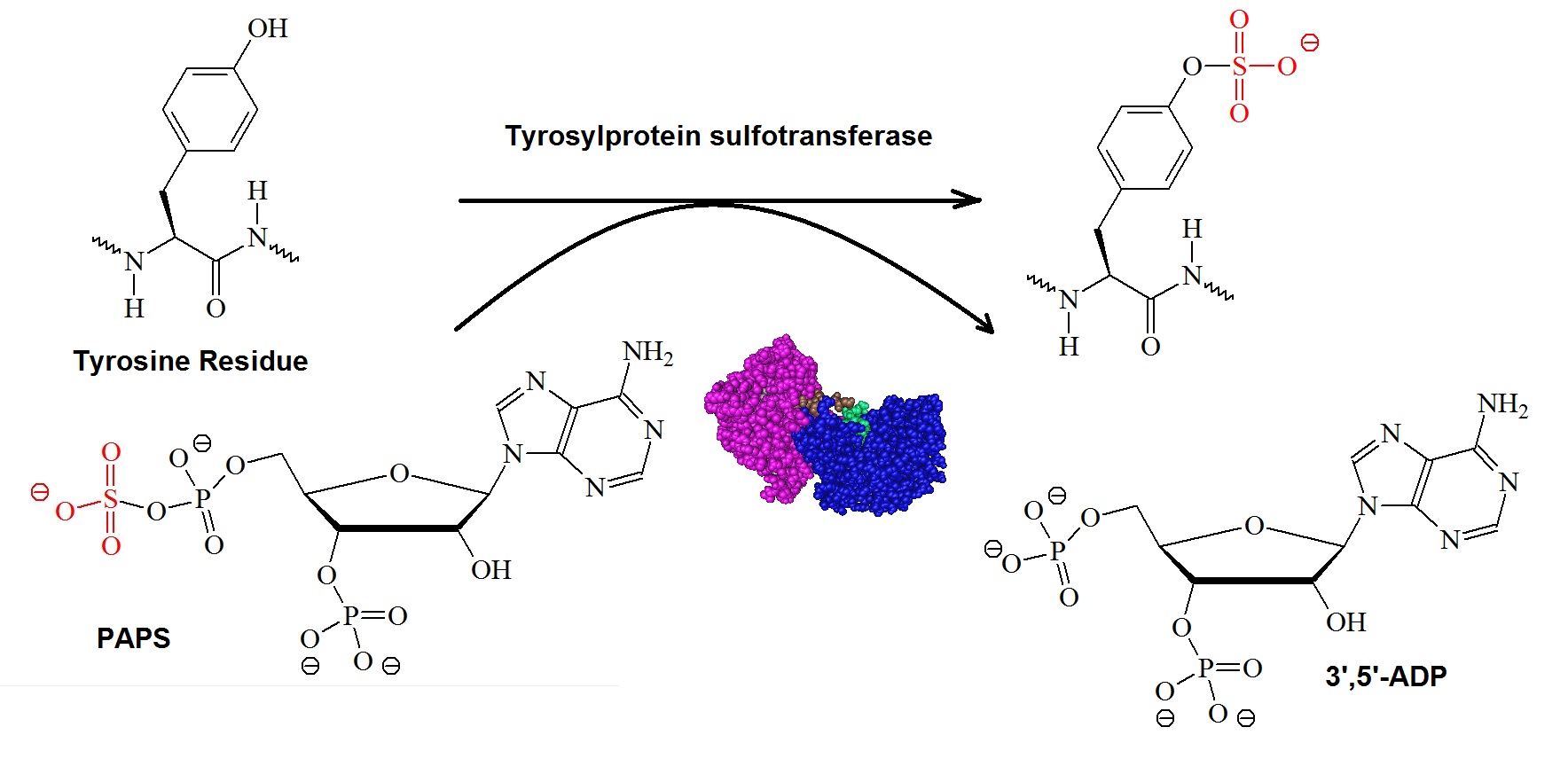
Figure 1: The tyrosylprotein sulfotransferase reaction. The enzymes tyrosylprotein sulfotransferases or TPSTs (EC 2.8.2.20) catalyze the transfer of a sulfonate group from the donor compound 3’-phosphoadenosine 5’-phosphosulfate or PAPS to the hydroxyl group of a luminally oriented peptidyltyrosine residue to form a tyrosine O4-sulfate ester and 3’,5’-ADP.
Besset et al. reported in 2000 that human PAPS synthetase 1 (PAPSS1) accumulates in the nucleus of mammalian cells, and that PAPSS2 is localized in the cytoplasm. However, when PAPSS1 and PAPSS2 are coexpressed ectopically in mammalian cells, PAPSS2 is relocated to the nucleus. These results indicated that a sulfation pathway may exist in the nucleus of eukaryotic cells.
According to Kevin Moore, during sulfate activation and tyrosine O-sulfation inorganic sulfate enters the cell by the action of one of several sulfate transporters. Once the inorganic sulfate is in the cytosol it is then activated by the action of one of two PAPS synthases (PAPSS1 or PAPSS2). These bifunctional enzymes contain a C-terminal ATP sulfurylase domain and an N-terminal adenosine phosphosulfate (APS) kinase domain. First, ATP and inorganic sulfate are converted to APS and pyrophosphate by ATP sulfurylase. Next, APS is then channeled directly between the ATP sulfurylase and APS kinase active sites. The second step consumes a second ATP to phosphorylate the 3′-hydroxyl of the ribose ring of APS to yield PAPS and ADP. The reaction is catalyzed by the APS kinase domain. Next, PAPS is then transported into the Golgi lumen by a PAPS translocase. This transporter functions via an antiporter mechanism with PAP as the returning ligand. Once inside the Golgi lumen PAPS acts as the sulfate donor for TPSTs and all other carbohydrate sulfotransferases. The resulting sulfated products are either secreted or retained in the membrane of lysosomes, secretory vesicles, and/or the plasma membrane.
However, the functional involvement of protein tyrosine sulfation remains to be fully elucidated. Tyrosine sulfation appears to be important for the alteration of biological activities of proteins such as proteolytic processing of bioactive peptides, changes in the half-life of proteins in circulation, and modulation of extracellular protein-protein interactions including inflammatory leukocyte adhesion. However, the recent discovery of tyrosine sulfation of chemokine receptors suggests an even broader role in the inflammatory response.
For example, the sulfated tyrosine residues located in the N-terminal extracellular region of the chemokine receptor CCR5 have been shown to be important in mediating HIV binding and infection.
Teramoto et al. in 2013 reported the crystal structure of the human TPST isoform 2 (TPST2) complexed with a substrate peptide (C4P5Y3) derived from complement C4 and 3’-phosphoadenosine-5’-phosphate (PAP) at 1.9Å resolution. Their structural and complementary mutational analysis revealed the molecular basis for catalysis being an SN2-like in-line displacement mechanism. The enzyme TPST2 appeared to recognize the C4 peptide in a deep cleft by using a short parallel β-sheet type interaction. Furthermore, the bound C4P5Y3 forms an L-shaped structure. Apparently the mode of substrate peptide recognition observed in the TPST2 structure resembles the observed mode of recognition for the receptor type tyrosine kinases.
The peptide, E-5-D-4-F-3-E-2-D-1-Yacceptor-E+1-F+2-D+3, had been reported to be sulfated by the rat TPST with a Km of 5.4 μM. (Yacceptor represents the acceptor site for sulfation).
Synthetic peptides containing sulfotyrosine can be utilized as substrates for the study of sulfation kinetics. Simpson et al. in 2009 reported a peptide synthesis strategy for the synthesis of sulfotyrosine derivatives. The research group utilized the neopentyl protecting group for sulfate monoesters to achieve a high-yield synthesis of tyrosine-sulfated peptides.
Reference
Baeuerle PA, Huttner WB.; Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655-64.
STEVE BESSET, JEAN-BAPTISTE VINCOURT, FRANÇOIS AMALRIC and JEAN-PHILIPPE GIRARD; Nuclear localization of PAPS synthetase 1: a sulfate activation pathway in the nucleus of eukaryotic cells. February 2000 The FASEB Journal vol. 14 no. 2 345-354.
Falany, C. N. (1997) Sulfation and sulfotransferases. Introduction: changing view of sulfation and the cytosolic sulfotransferases. FASEB J. 11, 1–2.
Adam J. Hoffhines, Eugen Damoc, Kristie G. Bridges, Julie A. Leary, and Kevin L. Moore; Detection and Purification of Tyrosine-sulfated Proteins Using a Novel Anti-sulfotyrosine Monoclonal Antibody. THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 49, pp. 37877–37887.
Kanan Y, Al-Ubaidi MR (2013) Tyrosine O Sulfation: An Overview. JSM Biotechnol Bioeng 1(1): 1003.
Klaassen CD, Boles JW.; Sulfation and sulfotransferases 5: the importance of 3'-phosphoadenosine 5'-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J. 1997 May;11(6):404-18.
Kevin L. Moore; The Biology and Enzymology of Protein Tyrosine O-Sulfation The Journal of Biological Chemistry, (2003) 278, 24243-24246.
Levi S. Simpson, John Z. Zhu, Theodore S. Widlanski, and Martin J. Stone; Regulation of Chemokine Recognition by Site-Specific Tyrosine Sulfation of Receptor Peptides. Chem Biol. 2009 February 27; 16(2): 153–161. doi:10.1016/j.chembiol.2008.12.007.
Teramoto T, Fujikawa Y, Kawaguchi Y, Kurogi K, Soejima M, Adachi R, Nakanishi Y, Mishiro-Sato E, Liu MC, Sakakibara Y, Suiko M, Kimura M, Kakuta Y; Crystal structure of human tyrosylprotein sulfotransferase-2 reveals the mechanism of protein tyrosine sulfation reaction. Nat Commun (2013) 4 p.1572.