MARIO refers to a method or technology called
“Mapping RNA interactome in vivo."
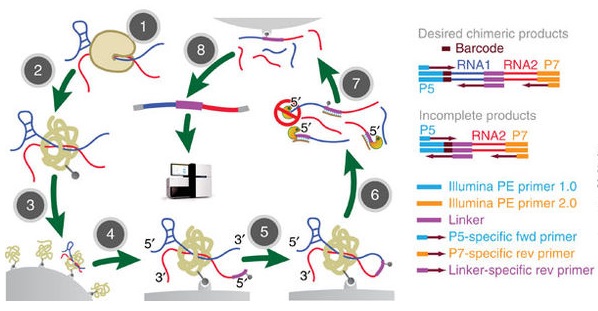
MARIO reveals RNA–RNA interactions from unperturbed cells. (Nguyen et al. 2016)
(1) MARIO allows the direct analyses of endogenous cellular features without
introducing any exogenous nucleotides or protein-coding genes before
cross-linking.
(2) Introduction of a selectable linker enables an unbiased selection of interacting
RNAs. MARIO enables global mapping of an RNA–RNA interactome.
(3) MARIO only captures the RNA molecules co-bound with a single protein molecule.
The capture of RNA molecules that are independently bound to different copies of
a protein is avoided.
(4) False positives resulting from RNAs ligating randomly to other nearby RNAs are
minimized by performing the RNA ligation step on streptavidin beads in extremely
dilute conditions.
(5) The RNA linker used provides a clear boundary delineating the position of ligation
site in the sequencing reads, avoiding ambiguities in mapping the ligated chimeric
RNA.
(6) Potential PCR amplification biases are removed by attaching a random four-
or six-nucleotide barcode to each chimeric RNA before PCR amplification and
subsequently counting completely overlapping sequencing reads with identical
barcodes only once.
[Source: Nguyen et al. 2016]
What does MARIO do?
MARIO allows
(i) The capture of interacting RNAs in vivo in an unbiased manner without genetically
or transiently introducing exogenous molecules;
(ii) Stringent removal of non-physiologic associations that form after cell lysis;
(iii) Selects proximity-ligated chimeric RNAs; and
(iv) Unambiguous bioinformatic identification of interacting RNAs
How does MARIO work ?
MARIO starts with the cross-linking of RNAs to proteins in cells. The major experimental steps needed are as follows:
(1) Cross-linking and immobilization of all RNA–protein complexes in streptavidin
beads and removal of nonspecific binding by denaturing conditions;
(2) RNA fragmentation, protein denaturing and biotinylation;
(3) Immobilization of RNA-binding proteins at low density;
(4) Ligation of a biotinylated RNA linker to facilitate selective enrichment of
chimeric RNA constructs. The linker sequence splits the interacting RNAs
from a sequencing read pair.
A biotin-tagged RNA linker is attached to the RNA’s 5′-end
(5′- rCrUrArG/iBiodT/rArGrCrCrCrArUrGrCrArArUrG rCrGrArGrGrA -3′);
(5) Proximity ligation under a dilute condition;
(6) RNA purification and RT;
(7) Biotin pull-down; and
(8) Construction of sequencing library.
What does MARIO reveal?
(A) MARIO maps RNA–RNA interactions in a massive scale.
(B) MARIO can identify protein-assisted between-molecule and within-molecule
RNA interactions.
(C) The MARIO identified RNA–RNA interactome is composed of tens of thousands of
interactions, which involve mRNA, long intergenic noncoding RNA (lincRNA),
small nucleolar RNA (snoRNA), small nuclear RNA, tRNA, miRNA, transposon
RNA, pseudogene RNA, antisense RNA and novel transcripts.
(D) The identified RNA–RNA interactome is a scale-free network.
(E) Long non-coding RNA including lincRNA, transposon RNA, and pseudogene RNA
are observed to interact with mRNA.
(F) Sequence complementation is observed in interactions between transposon
(LINE and LTR) RNA and mRNA, as well as in mRNA–mRNA,
mRNA–peudogene RNA, lincRNA–mRNA, miRNA–mRNA and LINE–miRNA
interactions.
(G) MARIO data also provide spatial-proximity information related to RNA folding in
three-dimensional space.
Examples of oligonucleotide sequences used for MARIO
Custom-designed RNA and DNA oligonucleotides used in the procedure are listed below.
(All oligonucleotides used are free of RNase and are HPLC-purified):
|
|
5′- ------------------------------------------------------------------- -3′
|
|
Biotinylated RNA linker
|
rCrUrArG/iBiodT/rArGrCrCrCrArUrGrCrArArUrGrCrGrArGrGrA
|
|
cDNA strand with RNA linker
|
T*C*G*C*ATTGCATGGGCTACTAGCAT
* denotes phosphor thioate bonds to block digestion by T7 exonuclease.
|
|
Pre-adenylated RT adapter
|
/5rApp/AGATCGGAAGAGCGGTTCAG/3ddC/
|
|
|
|
|
Primer for the ES-1 sample:
|
/5Phos/NNAGGTNNNAGATCGGAAGAGCGTCGTGgatcCTGAACCGCTCTTCCGATCT
|
|
RT Primer for the ES-2 and MEF samples (sequenced on different lanes):
|
/5Phos/NNCGCCNNNNAGATCGGAAGAGCGTCGTGgatcCTGAACCGCTCTTCCGATCT
|
|
RT Primer for the ES-indirect sample:
|
/5Phos/NNCATTNNNNAGATCGGAAGAGCGTCGTGgatcCTGAACCGCTCTTCCGATCT
|
|
Cut_oligo
|
GTTCAGGATCCACGACGCTCTTCAAAA/3InvdT/.
BamHI restriction site is highlighted
|
|
Truncated PCR Forward Primer DP5
|
CACGACGCTCTTCCGATCT
|
|
Truncated PCR Reverse Primer DP3
|
CTGAACCGCTCTTCCGATCT
|
|
Illumina PE PCR Forward Primer 1.0
|
AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT
|
|
Illumina PE PCR Reverse Primer 2.0
|
CAAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGCTGAACCGCTCTTCCGATCT
|
Reference
Nguyen, Tri C., Cao, Xiaoyi, Yu, Pengfei, Xiao, Shu, Lu, Jia, Biase, Fernando H., Sridhar, Bharat, Huang, Norman, Zhang, Kang, and Zhong, Sheng; Mapping RNA-RNA interactome and RNA structure in vivo by MARIO. Nature Communications 2016,7, 12023.
---...---