Various acidic and basic functional groups on amino acid side chains and termini of proteins and Peptides may be charged. The presence of a charge is dependent on the pKa values of the groups and the pH of the solution.
The isoelectric point (pI) of a peptide is defined as the pH at which the peptide has a net charge of zero.
-
At pH values below the pI, peptides carry a net positive charge;
-
Above the pI, they carry a net negative charge
pI-values of peptides can be utilized as a basis for separation in solution. If a voltage is applied to a complex peptide mixture in a pH gradient, the peptide will migrate to the pH at which they are neutrally charged. The pI of a peptide maybe accurately calculated from its sequence, and provides information that complements mass spectrometric methods for protein and peptide identification.
The pI as well as net charges of peptides can be calculated using amino acid information either manually or with the help of a peptide calculator. An example for the peptide with the sequence "PEPTIDE" is shown below.
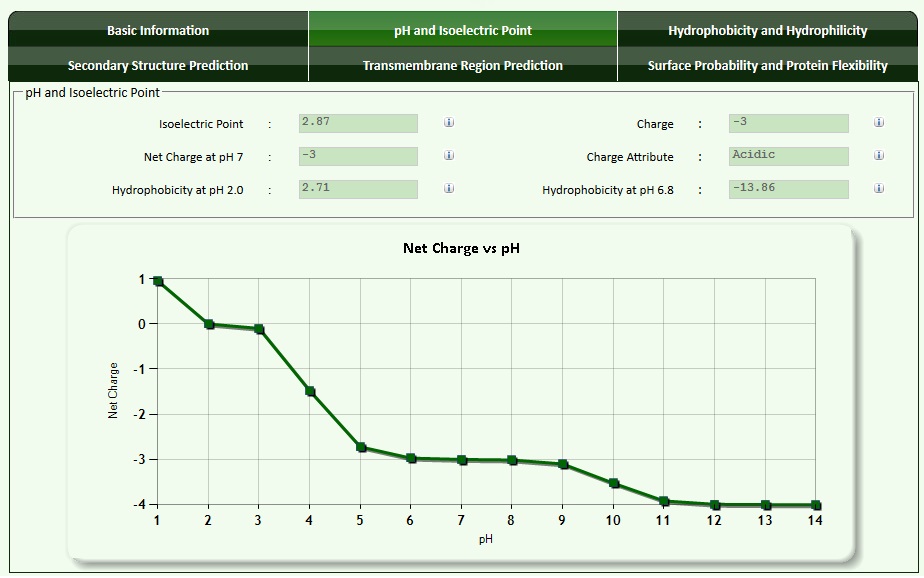
The next graphic shows basic information for the peptide.
.jpg)
Additional Information:
To learn more about isoelectric points, click here!