Thiol-modifiers enable covalent attachment of oligonucleotides to a variety of biological molecules such as ligands, peptides or proteins, as well as solid supports.
Thiol modifiers (-SH) allow the covalent attachment of oligonucleotides to a variety of ligands or supports during or after solid phase synthesis of DNA or RNA oligonucleotides. A thiol-modifier can be used as an alternative for an amino group. The modifier can be placed at either the 5’-end or the 3’-end of an oligonucleotide. This modifier is a useful functional group for the formation of a reversible disulfide bond or an irreversible sulfide bond which opens the possibility of specific coupling to sulfhydryl containing ligands by forming a disulfide bridge. Activated esters or isothiocyanate derivatives are often used for adding the thiol group to free amino-modified oligonucleotides. Also, thiol modifiers can be used to conjugate oligonucleotides to proteins or peptides.
Maleimide, bromide, iodide, or sulphonyl derivatives are also suitable for conjugating thiol-linked oligonucleotides to a variety of functional groups such as fluorescent dyes, biotin, or alkaline phosphatase. The conjugation of oligonucleotides to enzymes such as alkaline phosphatase or horseradish peroxidase is commonly used in the production of molecular probe systems or assays. Attachment of oligonucleotides to solid supports or surfaces can be achieved via disulfide bonds or maleimide linkages. After synthesis of the final product, HPLC purification is recommended to achieve accurate data during biological or chemical experiments.
A selection of thiol-modifier phosphoramidites is commercially available.
Thiol-modifier phosphoramidites
An oligonucleotide with thiol groups is an important intermediate in the production of fluorescent or other similar oligonucleotide conjugates. Applications for thiol modifiers are quite similar to amino-modifiers. Thiol groups containing phosphoramidites are used for the introduction of thiol groups into oligonucleotides. Often the thiol group is used to attach labels such as biotin or fluorescent tags to an oligonucleotide. Also, iodoacetate or maleimide derivatives are useful for the conjugation of other compounds via thioether linkages.
The thiol group is orthogonal to an amino group which allows adding of two or more functional groups to the same oligonucleotide enabling the conjugation of more than one tag.
5’-Thiol Modifiers
The thiol modifier S-trityl-6-mercaptohexyl-1-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite is used for the incorporation of a thiol group at the 5’-end of an oligonucleotide.
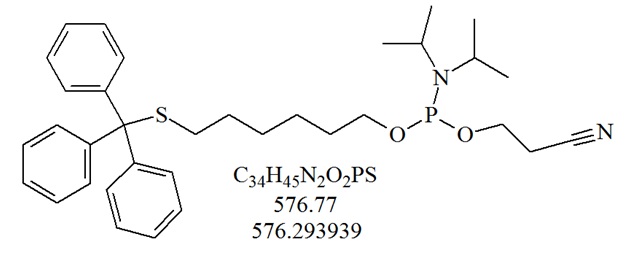
Structure of thiol-modifier S-trityl-6-mercaptohexyl-1-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite. The protecting trityl group can be removed with silver nitrate and dithiothreitol (DTT).
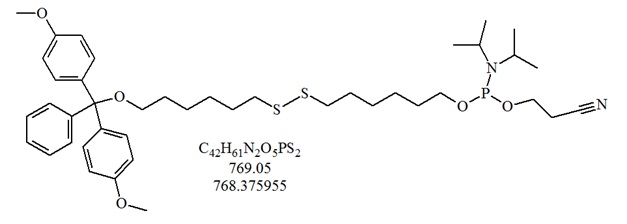
Structure of a the thiol-modifier 1-O-Dimethoxytrityl-hexyl-disulfide,1’-[(2-cyanoethyl)-(N,N-diisopropyl)]-phophoramidite. Here the disulfide can be cleaved after normal deprotection at room temperature with 100 mM DTT at pH 8.3 to 8.5 in 30 minutes to release a free thiol group.
3’-Thiol Modifier
Thiol modifier supports with three or six atom spacers are commonly used for the introduction of thiol modifiers on the 3’-end of oligonucleotides. The 3’-thiol modifier C3 S-S CPG is routinely used for the introduction of a thiol group to the 3’-terminal end of the target oligonucleotide.
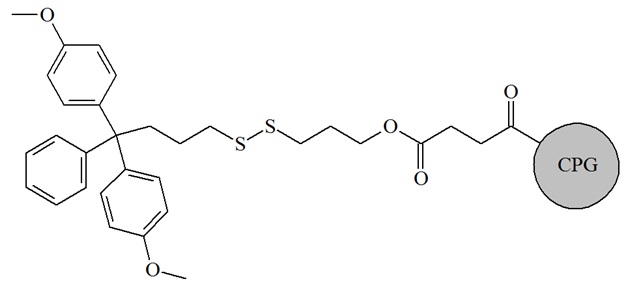
Structure of thiol-modifier C3 S-S CPG or 1-O-Dimethoxytrityl-propyl-disulfide,1’-succinyl-lcaa-CPG. After normal deprotection, the disulfide can be cleaved at room temperature within 30 minutes by adding 100 mM DTT to a buffer at pH 8.3 to 8.5.
Thioctic acid derivatives can be used for the modification of oligonucleotides that are useful for the immobilization of oligonucleotides to solid supports, generally used for the design and production of microarrays, biosensors or similar devices. Thiol based linkers have a natural affinity to metals and are therefore useful modifications to conjugate oligonucleotides to gold and silver surfaces or particles including gold based nanodots.
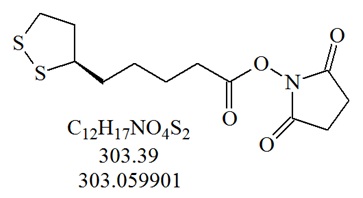
Structure of Thioctic Acid NHS ester. After cleavage of modified oligonucleotides this activated thiol compound can be attached to the 3’-end using a 3’-modified solid support, or the 5’-end post-synthetically using an amino-modified oligonucleotide. Oligonucleotides containing this moiety can be directly attached to gold or silver surfaces.
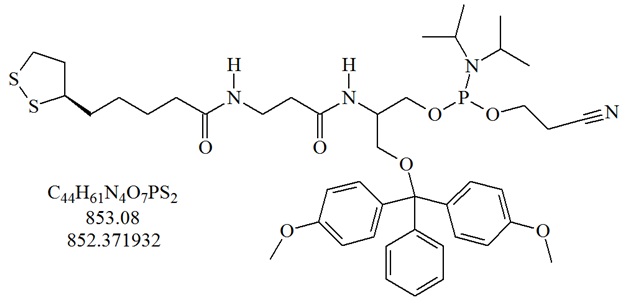
Structure of dithiol serinol phosphoramidite, {3-Dimethoxytrityloxy-2-(3-((R)-α-lipoamido)propanamido)propyl-1-O-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite}.
Gold nanoparticles are versatile tools for the design and production of biological sensors. Gold nanoparticles offer several options for functionalization and are therefore used for the production of sensors that allow for fluorescence, electrochemical, and similar detection techniques. Oligonucleotides, including aptamers, containing thiol groups can be used to functionalize gold nanoparticles. Dithiols or polythiols provide a higher level of stability and are therefore ideally suited for modifications of gold nanoparticles or surfaces.
A dT phosphoramidite modified with a spacer and a thiol group can is used for adding an internal thiol modified group to oligonucleotides. The S-Bz-Thiol-Modifier C6-dT is used for the incorporation into a nucleotide chain.
Internal Thiol Modifier
A deoxythymidine (dT) modified with a spacer, and a thiol group can be used for adding internal thiol modifier groups at desired locations within oligonucleotide sequences. This thiol modifier reacts directly with maleimides or haloacetamides.
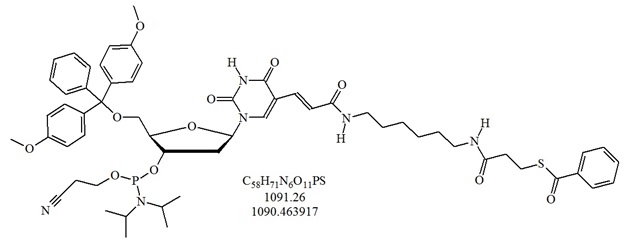
Structure of S-Bz-Thiol-Modifier C6-dT. 5'-(4,4'-Dimethoxytrityl)-5-[N-(6-(3-benzoylthiopropanoyl)- aminohexyl)- 3-acrylamido]-2'-deoxyuridine, 3'-[(2-cyanoethyl)- (N,N-diisopropyl)]-phosphoramidite.
Thiol-Exchange Reactions
Thiol-exchange reactions are useful for the production of cleavable conjugates. Here, disulfide bonds are created between the desired conjugates that can be cleaved using reducing reagents. Furthermore, the reducing environment in cells or tissue will also cleave these conjugates. Also, disulfide bonds can be cleaved chemically in vitro with commonly used reducing agents mercaptoethanol (2-ME), dithiothreitol (DTT), or tris(2-carboethyl)phosphine (TCEP). Also, 2-pyridyldithiol reagents are widely used thiol-exchange cross-linkers.
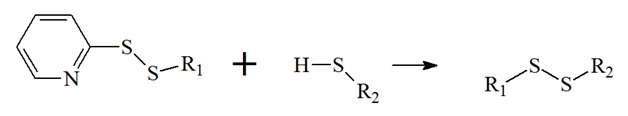
Thiol-exchange reaction of 2-pyridyldithiol based cross-linkers.
Reference
Pon and Yu; Linker phosphoramidite reagents for the attachment of the first nucleoside to underivatized solid-phase supports. Nucleic Acid Res. 2004: 32(2): 623-631. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC373346/
Books
Oligonucleotide Synthesis: Methods and Applications / Edition 1 by Piet Herdewijn
https://www.barnesandnoble.com/w/oligonucleotide-synthesis-piet-herdewijn/1101312233?ean=9781588292339&pcta=n&st=PLA&sid=BNB_DRS_Core+Shopping+Textbooks_00000000&2sid=Google_&sourceId=PLGoP22&k_clickid=3x22
Modified Nucleosides: in Biochemistry, Biotechnology and Medicine / Edition 1 by Piet Herdewijn https://www.barnesandnoble.com/w/modified-nucleosides-piet-herdewijn/1101190148?ean=9783527318209
---...---