First, let’s look how Science defines the term mass: The noun “mass” may describe “a body of coherent matter” which can be of indefinite shape and size. However, in the physical sciences, the quantity of matter is determined from its weight or from Newton’s second law of motion. Read more about this at: https://www.dictionary.com/browse/mass.
Whereas in mass spectrometry assigning a numerical value to the intrinsic property of “mass” is based on using carbon-12, 12C, as a reference point. Here, one unit of mass equals one Dalton (Da). One Dalton is defined as 1/12 the mass of a single carbon-12 atom.
Thus, one 12C atom has a mass of 12.0000 Da.
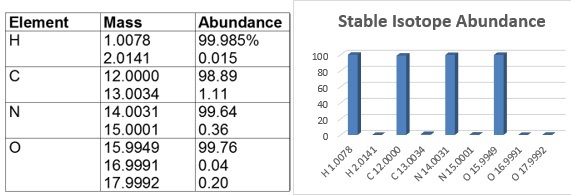
But there are also “Isotopes.” Most elements have more than one stable isotope.
For example, most carbon atoms have a mass of 12 Da, but in nature, 1.1% of C atoms have an extra neutron, making their mass 13 Da.
Why do we care?
Mass spectrometers can “see” isotope peaks if their resolution is high enough. A higher resolution, allowing to resolve isotopes mass peaks, results in better mass accuracy. Mass spectrometry is now one of the most critical analytical method used in analytical chemistry, biology, biotechnology, oligonucleotide chemistry, peptide chemistry as well as metabolomics and proteomics, among others.
The following examples use peptides to explain how mass spectrometry defines “mass.”
Table 1: Stable isotopes of most abundant elements present in peptides.
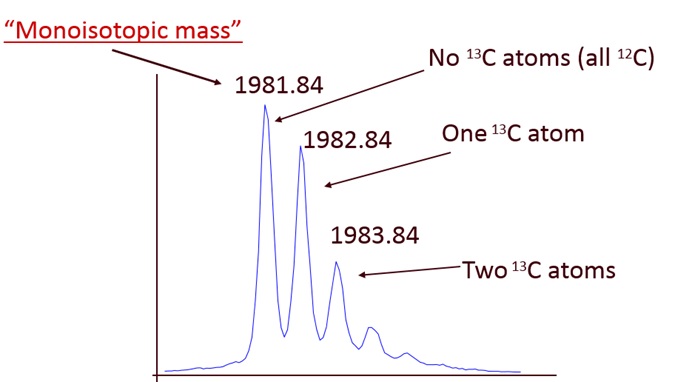
Example 1: A mass spectrum of a peptide with 94 carbon (C) atoms (19 amino acid residues)
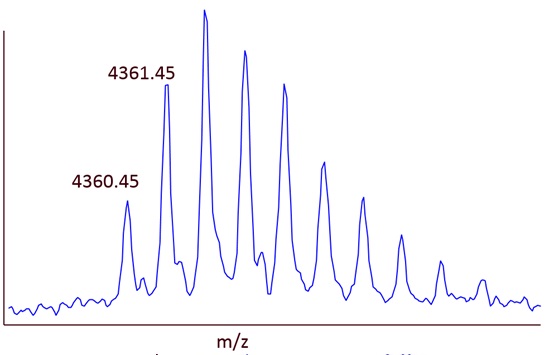
Example 2: Isotope pattern for a larger peptide (207 Carbon-atoms)
Example 3: Mass spectrum of insulin
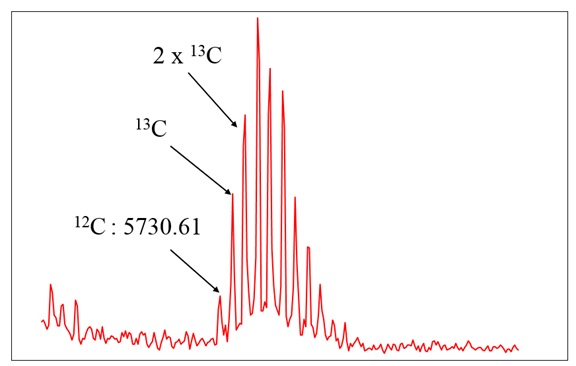
Insulin has 257 C-atoms. Above this mass, the monoisotopic peak is too small to be very useful, and the average mass is usually used.
Monoisotopic Mass
Example 4: Mass spectrum illustrating monoisotopic mass
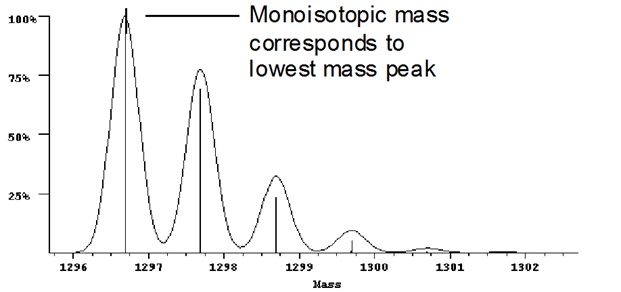
When the isotopes are clearly resolved the monoisotopic mass is used as it is the most accurate measurement.
Average Mass
Example 5: Mass spectrum illustrating average mass
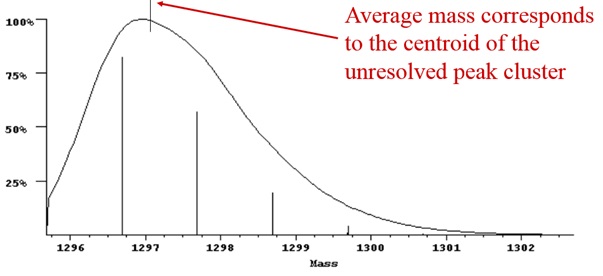
When the isotopes are not resolved, the centroid of the envelope corresponds to the weighted average of all the isotope peaks in the cluster, which is the same as the average or chemical mass.
Example 6: Nominal Mass, Monoisotopic Mass, and Average Mass
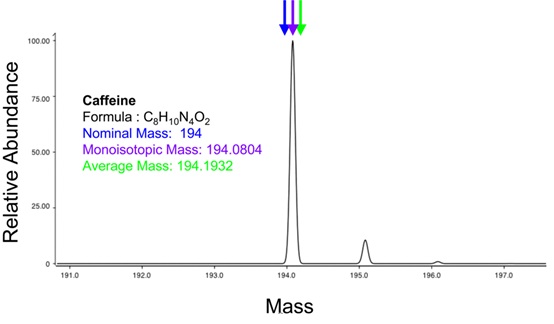
Theoretical isotope distribution for the molecular ion of caffeine (From Wikimedia Commons, the free media repository). The differences for nominal mass, monoisotopic mass, and average mass are illustrated.
Example 7: Theoretical Isotope Distribution for Glucagon
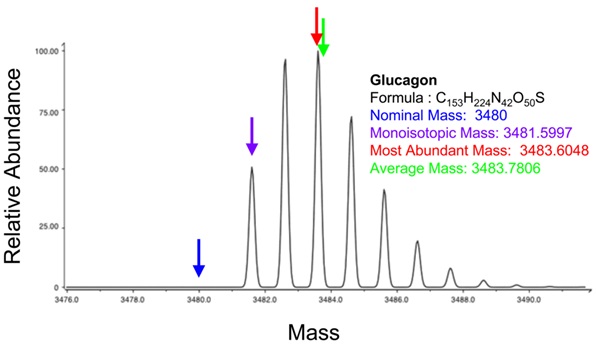
Theoretical isotope distribution for the molecular ion of the peptide glucagon (From Wikimedia Commons, the free media repository). The differences for nominal mass, monoisotopic mass, and average mass are illustrated. As the molecule becomes larger in mass the differences become clearer.
What to do if the resolution of the ion peaks in a mass spectrometer is not that good?
Example 8: Low or Poor Mass Resolution
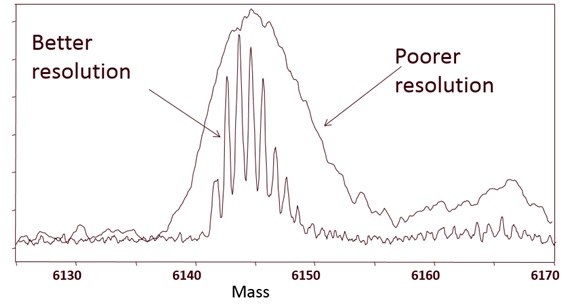
At lower resolution, the mass measured is the average mass!
---...---