To increase solubility and effectiveness of Paclitaxel, the conjugation of the drug to gold nanoparticles via DNA linkers is possible. According to Zhang et al., resulting conjugates usually show a 50-fold increase in solubility in aqueous buffers in comparison to the unconjugated drug. For visualization of the conjugation reaction and the effect of the drug conjugate in cells, the DNA linker can be labeled with fluorophores as well (Zhang et al. 2011). The final nano-gold DNA Paclitaxel conjugate represents a multimodal drug delivery system with
(i) enhanced solubility of the drug in aqueous systems, and serum-containing
cell culture media,
(ii) increased drug efficacy in Paclitaxel-resistant cells, and
(iii) a useful way of tracking the delivery of the drug.
However, other types of Paclitaxel conjugates are also possible.
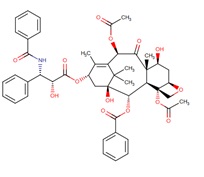
Taxol
|
.
|
Paclitaxel, Taxol A, (Source: Drugbank)
(2AR-(2aalpha,4beta, 4abeta,6beta, 9alpha(alpha r*,betas*), 11alpha,12alpha,12balpha))-beta-(benzoylamino)-alpha- hydroxy-benzenepropanoic acid 6,12b-bis(acetyloxy)-12-(benzoyl-oxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester. C47H51NO14.
Mw: Average: 853.9061 ; Monoisotopic: 853.330955345
|
“Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It was discovered in a US National Cancer Institute program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani isolated it from the bark of the Pacific yew tree, Taxus brevifolia and named it taxol. Later it was discovered that endophytic fungi in the bark synthesize paclitaxel. When it was developed commercially by Bristol-Myers Squibb (BMS), the generic name was changed to paclitaxel and the BMS compound is sold under the trademark Taxol. In this formulation, paclitaxel is dissolved in Kolliphor EL and ethanol, as a delivery agent. A newer formulation, in which paclitaxel is bound to albumin, is sold under the trademark Abraxane”. [Wikipedia, Drugbank]
Taxol binds to microtubules in the cell and slows down cell division and growth. It does so by stabilizing microtubules thereby blocking the segregation of chromosomes resulting in cell death or cellular apoptosis. However, formulation of Taxol into a delivery system acceptable for human use proved difficult. According to the NIH, Taxol is the best-selling drug ever manufactured.
Different illustrations of the tubulin structure with highlighted GTP (non-exchangeable) bound to α-tubulin, GDP (exchangeable) bound to β-tubulin, and Taxol.
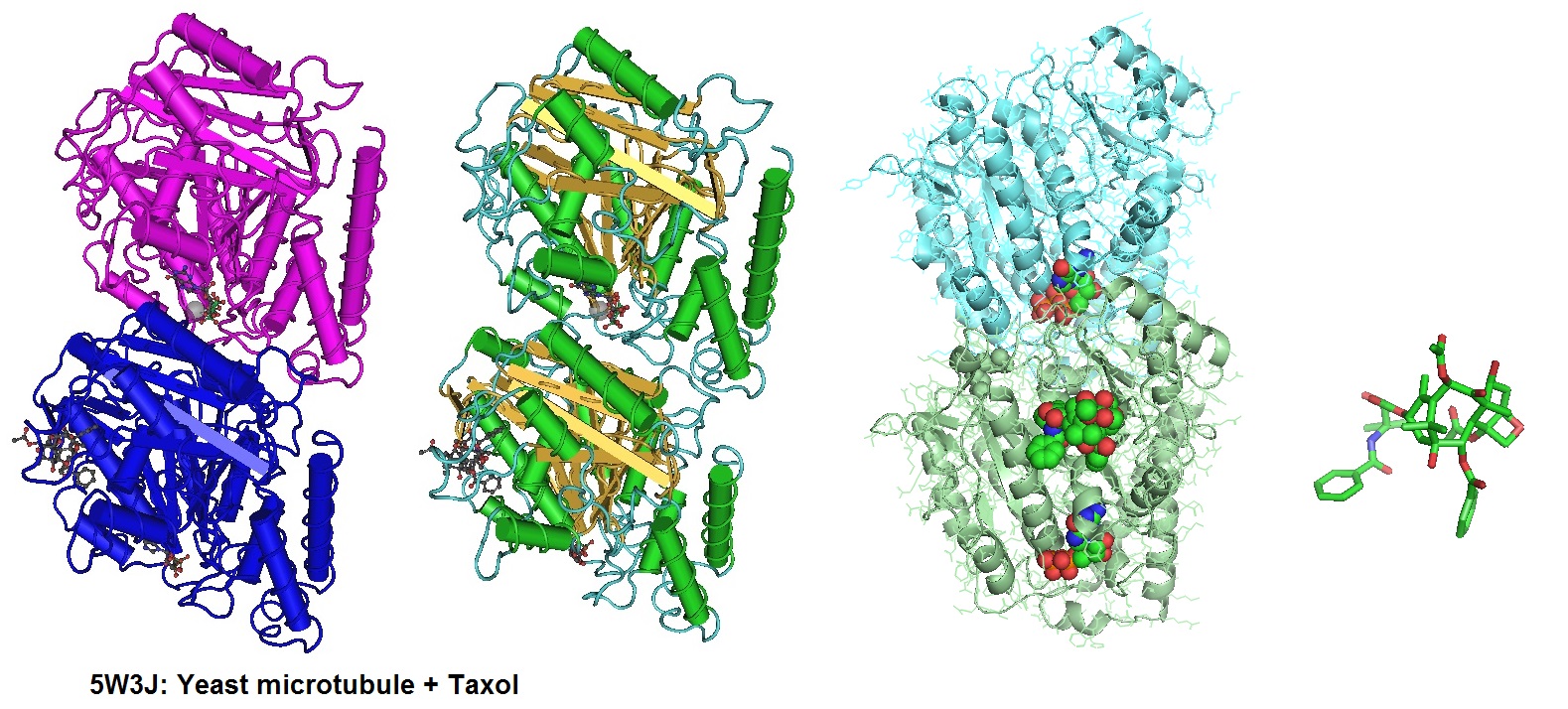
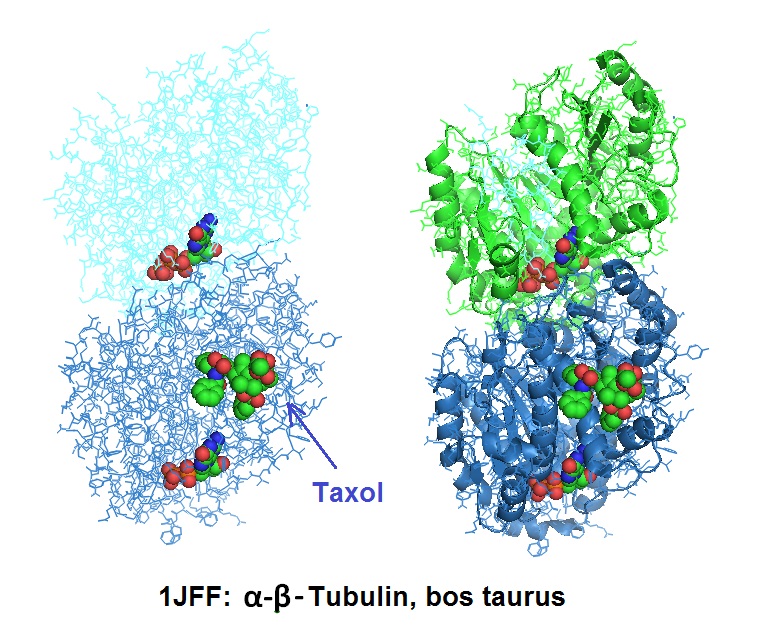
Due to its inherent insolubility in aqueous media, Paclitaxel can be limited in effectiveness. However, the covalent attachment to gold nanoparticles via DNA linkers results in conjugates highly soluble in aqueous buffers. Zhang et al. in 2011 reported a 50 fold increase in solubility of the conjugates when compared to the unconjugated drug.
The research group recommends the attachment of DNA-nanoparticle conjugates to drugs as a strategy for the enhancement of a wide variety of therapeutic agents that need to be formulated in aqueous media.
Reference
Hermanson, Grg T.; Bioconjugate Techniques.
Kim CK, Ghosh P, Rotello VM.; Multimodal drug delivery using gold nanoparticles. Nanoscale. 2009 Oct;1(1):61-7. doi: 10.1039/b9nr00112c. Epub 2009 Sep 4.
Zhang, X.-Q., Xu, X., Lam, R., Giljohann, D., Ho, D., & Mirkin, C. A. (2011). A Strategy for Increasing Drug Solubility and Efficacy through Covalent Attachment to Polyvalent DNA-Nanoparticle Conjugates. ACS Nano, 5(9), 6962–6970. http://doi.org/10.1021/nn201446c
---...---