Covalently modified oligonucleotides have many useful applications as bio-conjugate precursor molecules in biochemistry, experimental biology, biotechnology, molecular medicine, as well as other related research disciplines. Among the many possible conjugation approaches employed, the use of peptide linkers appears to be one of the most commonly utilized bio-conjugation attachment method that allow the creation of various unique biopolymers (1-3). This approach requires the availability of one biopolymer (or surface molecule) that is functionalized with one or more amino groups and a second molecule (or biopolymer) that is functionalized or activated with a carboxyl group. In this reaction type, oligonucleotide conjugation is carried out using a nucleophilic group located on the oligonucleotide that reacts with an electrophilic group present on a reporter molecule or a solid support. This is considered to be the predominant approach because the common oligonucleotide deprotection step is performed by base treatment using ammonia, primary alkylamines or both molecular types in various combinations. These molecules are inherently nucleophilic. However, sometimes researchers may want to introduce electrophilic groups to oligonucleotides in order to allow their attachment to nucleophilic moieties or surfaces that contain nucleophilic groups. When oligonucleotides have been used for this type of bio-conjugation reactions, the activated carboxylate, the salt or ester of the carboxylic acid, are usually generated post-synthetically (2). However, free carboxyl modified oligonucleotides can be activated using the water soluble cross-linker 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, EDAC or EDCI) in situ in organic or aqueous conditions and subsequently conjugated to an amino group carrying target compound or molecule. The reaction is illustrated in figure 1. Furthermore, in order to generate free carboxylate-attached-oligonucleotides using commercially available building blocks, the cleavage of the ester bond with an alkaline base is required before the oligonucleotide base deprotection step is carried out, using concentrated ammonia. Otherwise the ester will be converted into the corresponding amide (4, 5).
Figure 1.
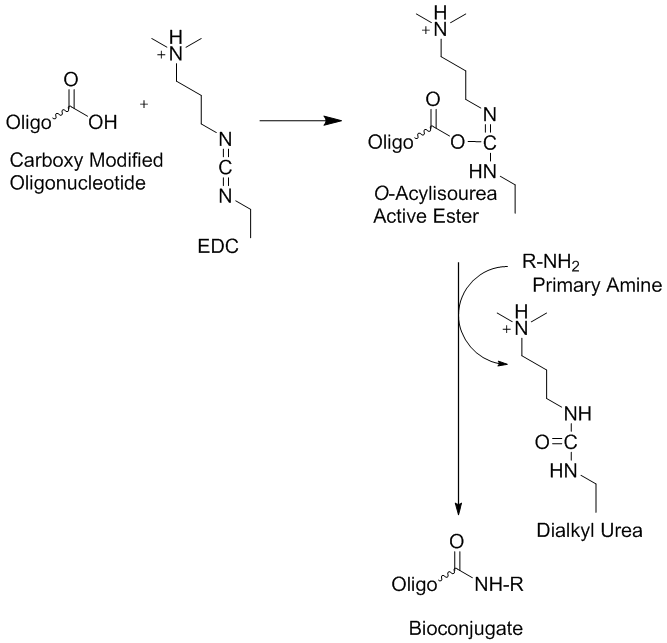
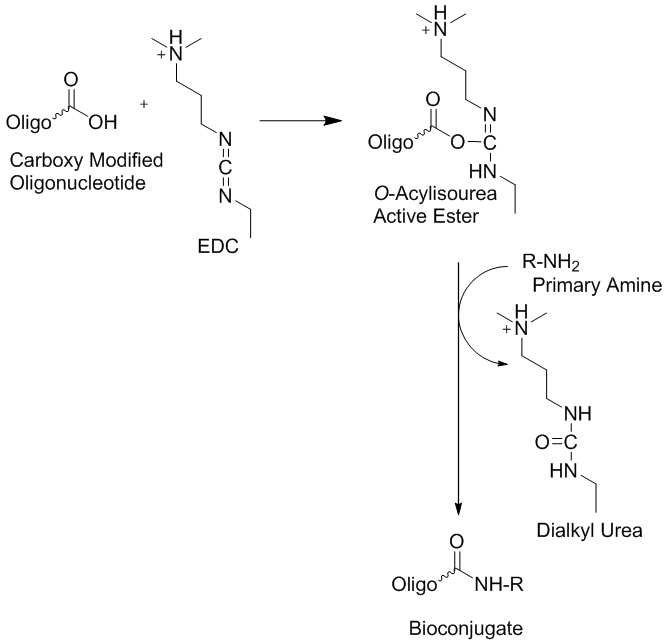
Figure 1: Reaction of free carboxyl modified oligonucleotides using the water soluble cross-linker 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, EDAC or EDCI) for the bio-conjugation to amino groups on target compounds or molecules that can also be located on solid supports such as microarrays.
The use of this strategy allows the immobilization of carboxyl modified oligonucleotides to solid supports such as micro-array slides and various types of aminoalkylated beads. These type of conjugates can be used to design and synthesize molecules that are cell permeable, can stabilize complexes with other oligonucleotides, have enhanced detectability relative to the respective unmodified compounds, as well as for the design of antisense oligonucleotides, 2’-O-methoxyethyl modified oligonucleotides sometimes also called gapmers, and others.
Bio-synthesis now offers a wide variety of 3’-, 5’- and internally carboxyl modified oligonucleotides as well as their conjugates to carbohydrates, peptides, proteins and antibodies (1).
References
1. http://www.biosyn.com/Bioconjugation.aspx
2. E. Jablonski, E. W.Moomaw, R. H.Tullis and J. L.Ruth Nucleic Acid Res., 1986, 14, 6115-6128.
3. J. D. Kahl and M. M. Greenberg J. Org. Chem., 1999, 64 (2), 507–510
4. A.V. Kachalova, T. S. Zatsepin, E. A. Romanova, D. A. Strelenko, M. J. Gait, T. S. Oretskaya Nucleosides, Nucleotides Nucleic Acids. 2000, 19, 1693-1707
5. T. P. Prakash, A. M. Kawasaki, E. A. Lesnik, S. R. Owens, M. Manoharan Org. Lett. 2003, 5, 403-406.