Temporins or temporin peptides are frog peptides that belong to a family of short hydrophobic peptides with antibacterial and antifungal properties. Temporins are 10 to 17 amino acids in length and contain an amide bond at the C-terminal end. Temporins are known to adopt a α-helical conformation in hydrophobic environments. Since temporins have the ability to perturb the integrity of cell membranes they are mostly effective against Gram-positive bacteria.
Figure 1: NMR structure of Temporin-1 TA in Lipopolysaccharide Micelles: FLPLIGRVLSGIL.
Because of the ‘lollipop’ like structure of temporins, temporins have become templates for the design and development of antibiotics. Moharan and Bhattachajya in 2016 reported the design and the structure of a ‘lollipop’-shaped helical hybrid antimicrobial peptide (LG21) with antibacterial activity. The hybrid peptide investigated consisted of temporin B (TB) and the β-lipopolysaccharide (LPS) binding motif. The researchers reported that the LPS binding motif of LG21 played dominant roles in broad spectrum activity.
The reported 3-D structure provided mechanistic insights for permeabilization of bacterial membranes. The hybrid peptide containing LPS binding motif is thought to be useful for structure based development of broad spectrum antibiotics.
Cationic antimicrobial peptides (AMPs) are a vital component of host innate immunity known for their lethal effects toward a variety of microorganisms. These include Gram-negative and Gram-positive bacteria, enveloped viruses, fungi, parasites and transformed or cancerous cells. As a mode of action, cationic AMPs bind and destabilize negatively charged bacterial membranes. However, some AMPs also target intra-cellular molecules such as nucleic acids, proteins and bacterial cell agglutination.
Simmaco and coworkers first used the term ‘temporin’ to describe a family of 10 structural related peptides with antibacterial and antifungal properties. The peptides were identified in electrically stimulated skin secretions of the European common frog Ran temporaria. Temporins are 10-13 amino acid residues in length and show some sequence similarity to hemolytic peptides isolated from Vespa venom. Natural and synthetic temporins are reported to have antibacterial activity against gram-positive bacteria. However, they are not hemolytic.
 The Common Frog (Rana temporaria) can be found throughout much of Europe. The picture to the left shows a fully-grown female.
The Common Frog (Rana temporaria) can be found throughout much of Europe. The picture to the left shows a fully-grown female.
(Source: https://commons.wikimedia.org/wiki/)
Further research has shown that the temporin family is widely distributed in ranid frogs originating in North America and Eurasia. In addition, temporin peptides have also been isolated from a few other related frogs.
Screening of a cDNA library prepared from the skin of R. temporaria using an oligonucleotide probe derived from the signal peptide region of preproesculentin-1 from R. esculenta allowed identification of the precursors of temporin B, temporin G, and temporin H.
Table 1: Temporin Peptides [Source: Simmaco et al. 1996]
|
Peptide
|
Sequence
|
|
Temporin A
|
FLPLIGRVLSGIL-NH2
|
|
Temporin B
|
LLPIVGNLLKSLL-NH2
|
|
Temporin C
|
LLPILGNLLNGLL-NH2
|
|
Temporin D
|
VLPIIGNLLNSLL-NH2
|
|
Temporin E
|
VLPIIGNLLNSLL-NH2
|
|
Temporin F
|
FLPLIGKVLSGIL-NH2
|
|
Temporin G
|
FFPVIGRILNGIL-NH2
|
|
Temporin H
|
LSP---NLLKSLL-NH2
|
|
Temporin K
|
LLP---NLLKSLL-NH2
|
|
Temporin L
|
FVQWESKFLGRIL-NH2
|
The structural organization is the same for these three peptides and consists of a signal peptide, an acidic propeptide, and the temporin peptide. Preprotemporin mRNAs are expressed in extradermal tissues of the frogs.
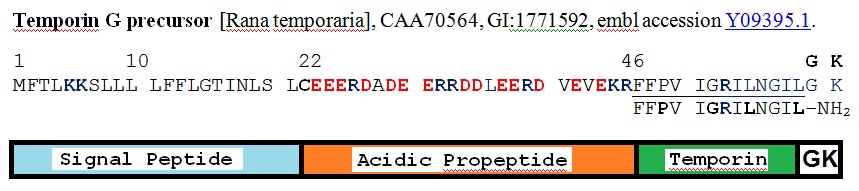
Figure 3: Schematic representation of the biosynthetic precursor of temoporin G from http://www.ncbi.nlm.nih.gov/protein/CAA70564.1. Cys 22 is the probable site of cleavage of the signal peptide, K 45 R 46 is the site of cleavage of a prohormone convertase, and G 60 act as the nitrogen donor for C-terminal amide formation.
Mangoni and coworkers found that temporins A and B had anti-Leishmania activity at micromolar concentrations with no cytolytic activity against human erythrocytes. Temporins are reported to be the shortest natural peptides with the highest leishmanicidal activity and the lowest number of positively charged amino acids that maintain biological function in serum.
According to the data reported by Mangoni et al. their mechanism of action involves plasma membrane permeation :
(i) Temporins induce a rapid collapse of the plasma membrane potential.
(ii) Temporins induce the influx of the vital dye SYTOX™ Green.
(iii) Temporins reduce intracellular ATP levels.
(iv) Temporins severely damage the membrane of the parasite.
Temporins have membranolytic effects that could make it difficult for the pathogen to develop resistance. Therefore temporins are potential candidates for the design of future antiparasitic drugs with a new mode of action. To determine minimal requirements for lytic efficiency and specific effects of temporins peptides Mangoni and coworkers studied effects of temporins A, B, and D against artificial membranes with different lipid composition and bacteria. Their results indicated that the lytic activity of temporins is not greatly affected by the membrane composition. Temporins A and B allowed leakage of large-size molecules from bacterial cells. Temporin H made the outer and the inner membrane of bacteria permeable to hydrophobic substances of low molecular mass. Temporin D had a cytotoxic effect on erythrocytes.
To conclude, temporins A, B and H change the permeability properties of bacterial membranes. Temporin D has a role in the animal defense. It is active against eukaryotic cells. These findings are thought to help designing new types of peptide based antibiotic drugs.
Reference
Mangoni ML, Rinaldi AC, Di Giulio A, Mignogna G, Bozzi A, Barra D, Simmaco M.; Structure-function relationships of temporins, small antimicrobial peptides from amphibian skin. Eur J Biochem. 2000 Mar;267(5):1447-54.
Maria Luisa Mangoni, José M. Saugar, Maria Dellisanti, Donatella Barra, Maurizio Simmaco and LuisRivas; Temporins, Small Antimicrobial Peptides with Leishmanicidal Activity. The Journal of Biological Chemistry 2005, 280, 984-990.
Mohanram H.; Nmr structure of temporin-1 Ta in lipopolysaccharide micelles: mechanistic insight into inactivation by outer membrane.
Mohanram H, Bhattacharjya S.; 'Lollipop'-shaped helical structure of a hybrid antimicrobial peptide of temporin B-lipopolysaccharide binding motif and mapping cationic residues in antibacterial activity. Biochim Biophys Acta. 2016 Jun;1860(6):1362-72. doi: 10.1016/j.bbagen.2016.03.025. Epub 2016 Mar 23.
Simmaco M, Mignogna G, Canofeni S, Miele R, Mangoni ML, Barra D.; Temporins, antimicrobial peptides from the European red frog Rana temporaria. Eur J Biochem. 1996 Dec 15;242(3):788-92.
Temporin G precursor [Rana temporaria]: http://www.ncbi.nlm.nih.gov/protein/CAA70564.