The recent pandemic caused by COVID-19 coronavirus has garnered unprecedented global effort to find appropriate therapeutics. Though our understanding of the underlying pathology is still incomplete, one arm of the endeavor has been to develop drugs to treat the infected. In the absence of cure, the other arm has been to develop vaccines to preempt the COVID-19 infection. Various types of vaccines are being considered employing different platforms: modified virus (attenuated or inactivated), nucleic acids (mRNA or DNA) to express viral protein, COVID-19 proteins, viral vectors expressing COVID-19 gene, etc (Dai et al., 2020; Rinaldi, 2020).
Unlike the conventional vaccines that require preparing a large titer of infectious viruses, nucleic acid vaccines could be prepared in a fraction of the time. This has prompted the development and clinical testing of the mRNA vaccines targeting the mutated spike protein of COVID-19 in a record time. At the time of writing of this article, FDA has issued EUA (emergency use authorization) for mRNA-based anti-COVID-19 vaccines developed by Moderna Therapeutics (USA) and Pfizer/BioNTech (USA/Germany). The key difference between the two vaccine preparations is the storage temperature (-20oC for Moderna's; -60 to -80oC for BioNTech's) required for preservation.
An ideal vaccine is expected to meet several objectives. The objectives may include blocking the spread of the virus, preventing symptoms associated with the infection, hospitalization, deaths, etc. Following the completion of preclinical testing on animals, clinical trials may begin after submitting an Investigational New Drug (IND) application and obtaining clearance from FDA. Normally, after completing Phase I, II and III studies, a new drug application (NDA) may be filed to FDA for market approval. During the unprecedented times like the COVID-19 pandemic, the above process may be bypassed in lieu of issuing EUA as a temporary measure.
In the case of Moderna Therapeutics, the process of developing mRNA vaccine began soon after learning the genomic sequence of COVID-19 in January, 2020. Phase I study (120 individuals; began March 16, 2020) assessed dose range, IgG antibody binding response by ELISA, and T cell response by intracellular cytokine stimulation assay, and safety of the mRNA-1273 vaccine. Upon finding that two consecutive injections of 100 ug or 250 ug dose (given 28 days apart) yielded similar clinical responses and that a lower reactogenicity (adverse reaction) was observed with 100 ug dose, the latter was selected to proceed to Phase II and III studies. Phase 2 study (600 individuals) examined the efficacy of 50 ug dose versus 100 ug dose and introduced saline solution as a placebo (negative control) using the previous dosing schedule. Additional laboratory studies assessed kidney function, liver function, blood count, coagulation, etc.
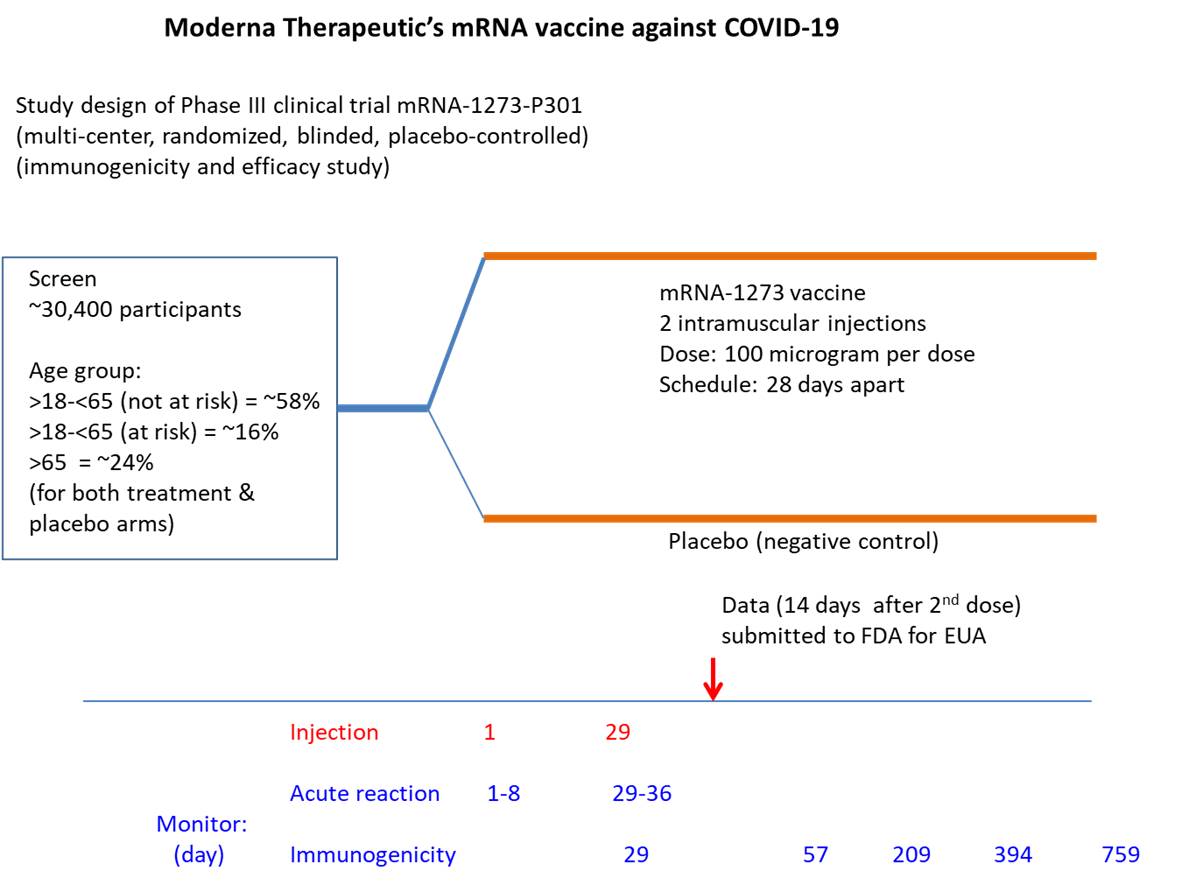
Phase III study included ~30,000 participants (47% female, 52% male) with the mean age of 51.4 (24.8% >65 y). As of 14 day post 2nd injection, the study found a higher percentage of COVID-19 infected cases in placebo (negative control) group (0.7%, 0.4%, 0.4% for age group 18-65y, 65-75y, >75y, respectively) than vaccine treated group (<0.1%, 0%, 0% for age group 18-65y, 65-75y, >75y, respectively). Though saline water was used as the placebo, an alternate vaccine targeting an irrelevant virus may have been more informative. Intriguingly, the study also showed similar efficacy for those with risk factors such as chronic lung disease, cardiac disease, obesity, liver disease, HIV infection (COVID-19 infection rate: 0.8% in placebo group, <0.1% for vaccine treated group). [https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-17-2020-meeting-announcement#event-materials ]. Phase I, II and III studies are still ongoing and may yield valuable information regarding side effects or long-term benefit. Some preliminary information for the vaccination of cancer patients is provided by the European Society for Medical Oncology (https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination).
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. For medicinal chemistry, it specializes in peptide synthesis, characterization, modification, purification to generate various peptide-based building blocks as well as pharmaceutical intermediates—in addition to peptide libraries, peptide arrays, peptidomimetics. Antibody purification, characterization/quantification, modification and labeling are also offered. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/tew/Messenger-RNA-(mRNA)-for-Vaccine-Development-Against-Coronavirus.aspx
https://www.biosyn.com/tew/Ribose-2’-O-methylation,-“self-and-non-self,”-and-Coronaviruses.aspx
https://www.biosyn.com/tew/RNA-Capping-and-De-Capping.aspx
References
Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 18:1-10 (2020). PMID: 33340022
Lindsay KE, Bhosle SM, et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging. Nat Biomed Eng. 3:371-380 (2019). PMID: 30936432
Rinaldi A. RNA to the rescue: RNA is one of the most promising targets for drug development given its wide variety of uses. EMBO Rep. 21:e51013 (2020). PMID: 32588530